Methods for characterizing and treating acute myeloid leukemia
A Myeloid Leukemia, Acute Technology Applied in the Improved Field of Treatment for AML Relapse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0318] Example 1: IMGN779 exhibits CD33-specific in vitro cytotoxicity against primary patient AML cells
[0319] CD33 levels and P-glycoprotein (Pgp) activity were measured by flow cytometry. Staining with WST-8 viability, the cytotoxic potency of DGN462 and IMGN779 in AML cell lines was assessed using serial exposure up to 7 days. The potency of IMGN779 was assessed against primary AML samples and normal bone marrow (NBM) using a colony formation assay after 24 hours of exposure and after long-term liquid culture to assess potency in leukemic progenitor and leukemic stem cells, respectively. The antitumor activity of IMGN779 was evaluated in SCID mice bearing subcutaneous HL60 / QC and EOL-1 xenografts.
[0320] Pharmacokinetic parameters in CD-1 mice were determined from plasma concentrations of IMGN779 conjugate and its total Z4681A antibody fraction at various time points measured by ELISA. The biological activity of a subset of these plasma samples was confirmed by assay...
Embodiment 2
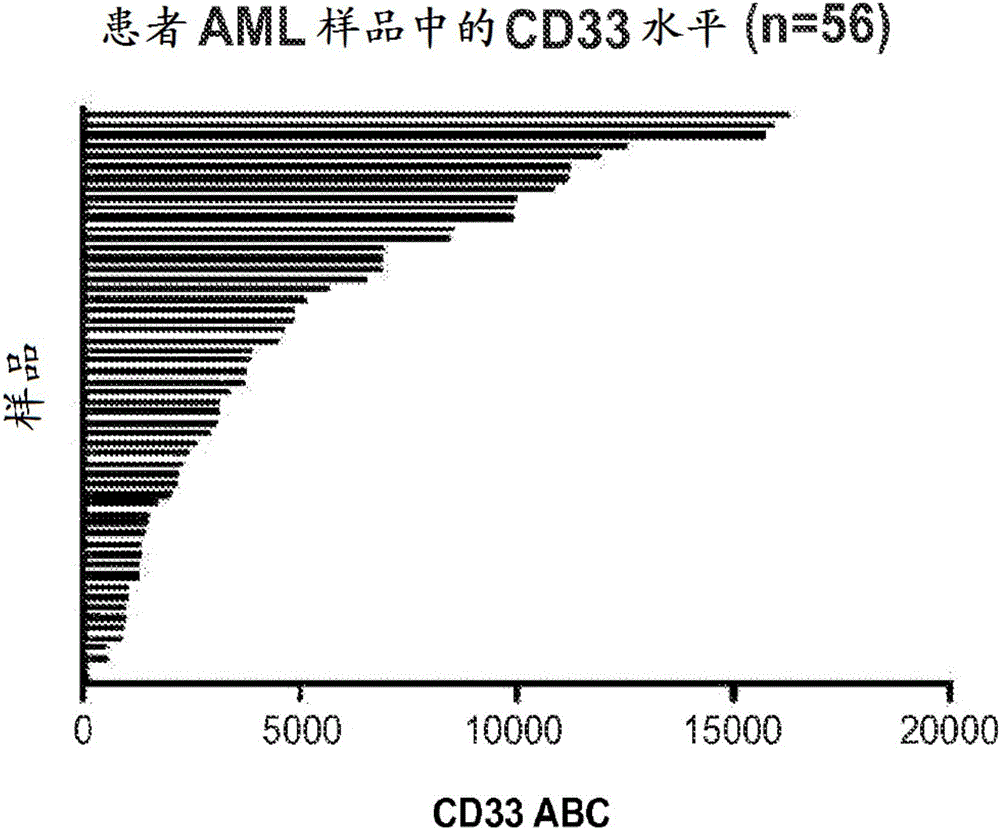
[0323] Example 2: CD33 Expression on Primary Patient AML Cells
[0324] CD33 expression on primary patient AML cells was measured using a calibrated flow cytometry method ( figure 1). Staining of AML samples with fluorescently-labeled anti-CD33 antibodies and comparison of the fluorescence signal with the calibration curve obtained using fluorescently-labeled beads at different marker-to-bead ratios allows the determination of the total number of CD33 antibodies bound per AML cell (ABC value ). CD33 is expressed at relatively low levels in patient AML cells, with a maximum expression of approximately 17,000 antigens per cell (ABC).
Embodiment 3
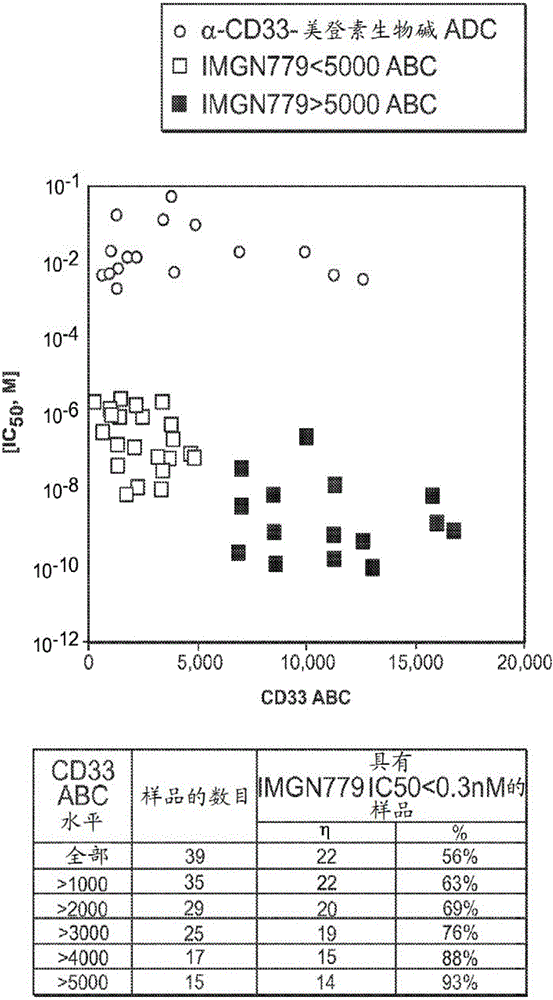
[0325] Example 3: IMGN779 demonstrates highly potent and CD33-specific in vitro cytotoxicity against primary patient AML cells.
[0326] The cytotoxic activity of IMGN779 was assessed against a panel of primary patient AML cells in a colony formation assay after 24 hours of conjugate exposure ( figure 2 ).
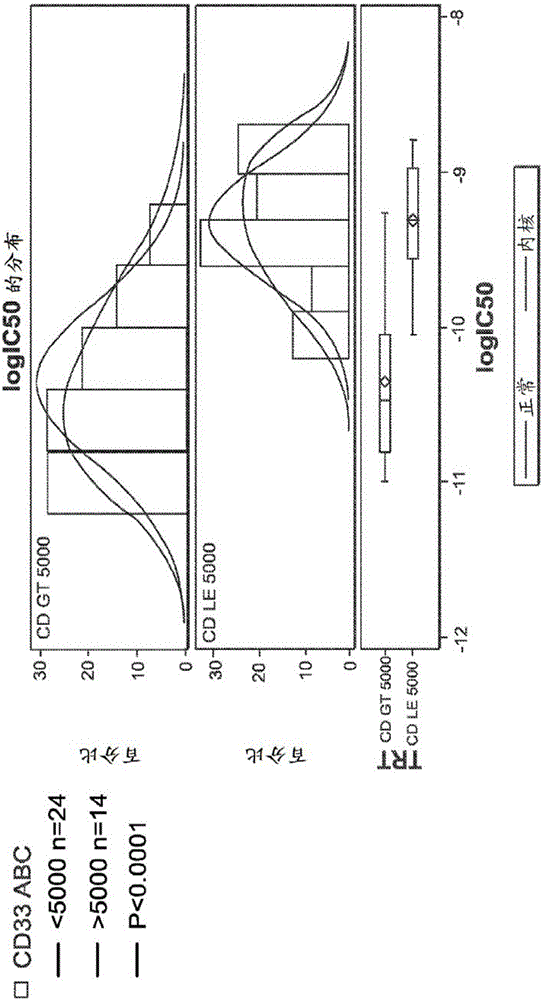
[0327] The activity of CD33-targeting maytansinoid ADCs (using the same antibody in IMGN779) was assessed on a subset of these samples. IMGN779 is highly active against patient AML cells, IC 50 Values range from 11 pM to 1.6 nM, dependent on CD33 expression level. CD33 levels range from approximately 200 to 16,000 antigens per cell. In comparison, CD33-targeting maytansinoid ADCs were 60 to 9,000-fold less active than IMGN779, independent of CD33 expression levels. figure 2 In vitro potency of IMGN779 compared to CD33-targeted maytansinoid ADC against patient AML cells is shown.
[0328] Use 0.3nM IC 50 Cut-off defines a high level of sensitivity (compared to the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com