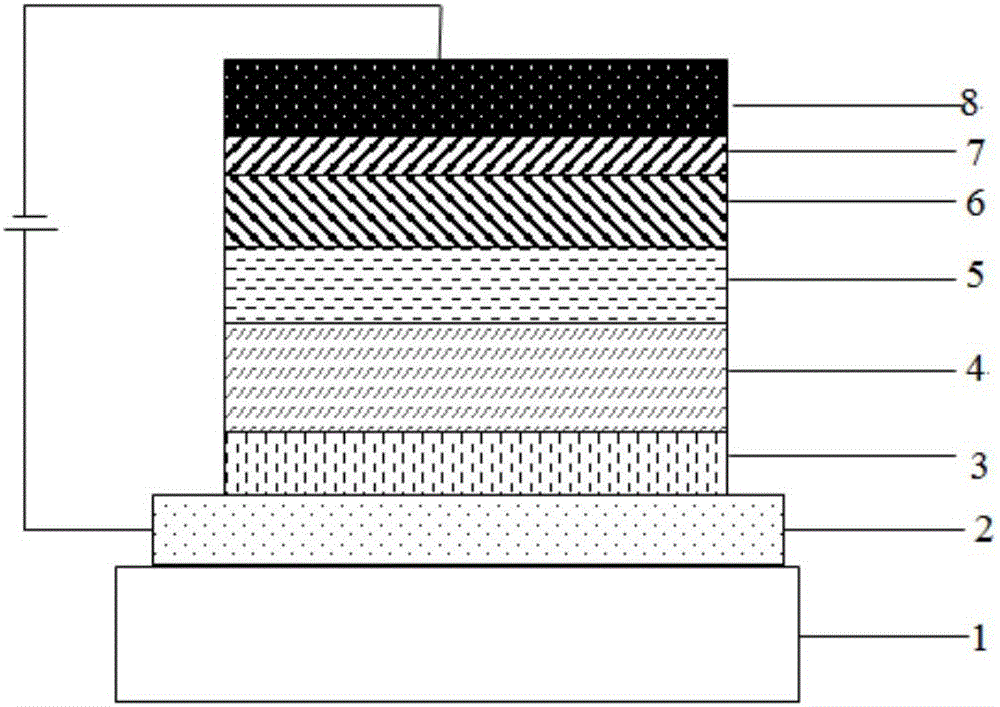
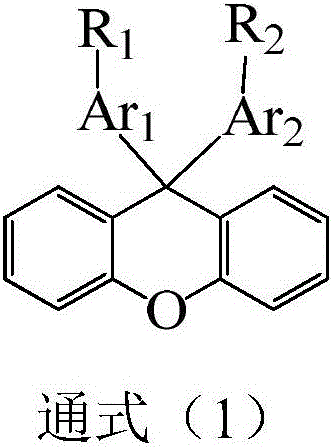
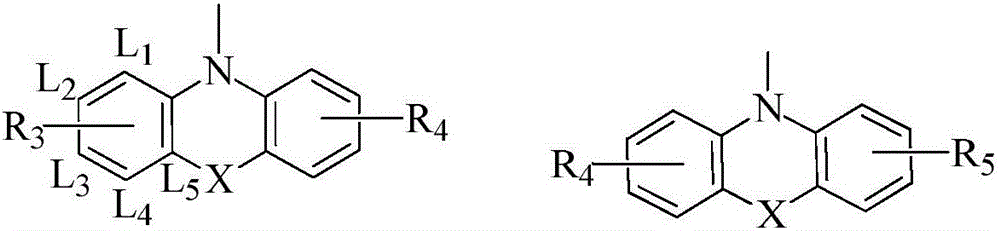Organic compound with xanthene and application thereof in OLED (organic LED) devices
An organic compound, xanthene technology, applied in the field of OLED, the field of compound materials containing xanthene structure as the central skeleton, can solve different problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1: the synthesis of compound 6:
[0073] synthetic route:
[0074]
[0075] In a 250ml three-neck flask, under the protection of nitrogen, add 0.01mol raw material A1, 0.012mol raw material B1, 0.03mol sodium tert-butoxide, 5×10 -5 molpd 2 (dba) 3 , 5×10 -5 mol of tri-tert-butylphosphine, 150ml of toluene, heated to reflux for 24 hours, sampling point plate, the reaction was complete; natural cooling, filtration, rotary evaporation of the filtrate, column chromatography to obtain the target product, HPLC purity 99.4%, yield 71.5%;
[0076] Elemental analysis structure (molecular formula C 53 h 36 N 2 o 2 ): theoretical value C, 86.86; H, 4.95; N, 3.82; 0, 4.37; test value: C, 86.87;
[0077] HPLC-MS: The molecular weight of the material is 732.87, and the measured molecular weight is 733.14.
Embodiment 2
[0078] Embodiment 2: the synthesis of compound 3:
[0079] synthetic route:
[0080]
[0081] In a 250ml three-neck flask, under the protection of nitrogen, add 0.01mol raw material A1, 0.012mol raw material B2, 0.03mol sodium tert-butoxide, 5×10 -5 mol pd 2 (dba) 3 , 5×10 -5 mol of tri-tert-butylphosphine, 150ml of toluene, heated to reflux for 24 hours, sampling point plate, the reaction was complete; natural cooling, filtration, rotary evaporation of the filtrate, column chromatography to obtain the target product, HPLC purity 99.1%, yield 72.5%;
[0082] Elemental analysis structure (molecular formula C 56 h 40 N 2 O): theoretical value C, 88.86; H, 5.33; N, 3.70; O, 2.11; tested value: C, 88.87; H, 5.32; N, 3.71;
[0083] HPLC-MS: The molecular weight of the material is 756.93, and the measured molecular weight is 757.31.
Embodiment 3
[0084] Embodiment 3: the synthesis of compound 10:
[0085] synthetic route:
[0086]
[0087] In a 250ml three-neck flask, under the protection of nitrogen, add 0.01mol raw material A1, 0.012mol raw material C1, 0.03mol sodium tert-butoxide, 5×10 -5 mol pd 2 (dba) 3 , 5×10 -5 mol of tri-tert-butylphosphine, 150ml of toluene, heated to reflux for 24 hours, sampling point plate, the reaction was complete; natural cooling, filtration, filtrate rotary evaporation, column chromatography to obtain the target product, HPLC purity 99.5%, yield 71.8%;
[0088] Elemental analysis structure (molecular formula C 58 h 40 N 2 o 2 ): theoretical value C, 87.41; H, 5.06; N, 3.52; O, 4.02; test value: C, 87.43;
[0089] HPLC-MS: The molecular weight of the material is 796.95, and the measured molecular weight is 797.38.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com