Preparation method of natural biflavones such as I3,II8-Biapgienin and Ridiculuflavone A
A biflavone, natural technology, applied in the preparation of new compounds and the field of medicine, can solve the problems of limited activity research, scarce sources, etc., and achieve the effect of a wide range of biological activities and simple purification methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] In order to understand the present invention, the present invention will be further described below in conjunction with embodiment; Following embodiment is illustrative, not limiting, can not limit protection scope of the present invention with following embodiment.
[0020] The present invention provides compounds 1 and 2
[0021]
[0022] The present invention specifically includes compounds:
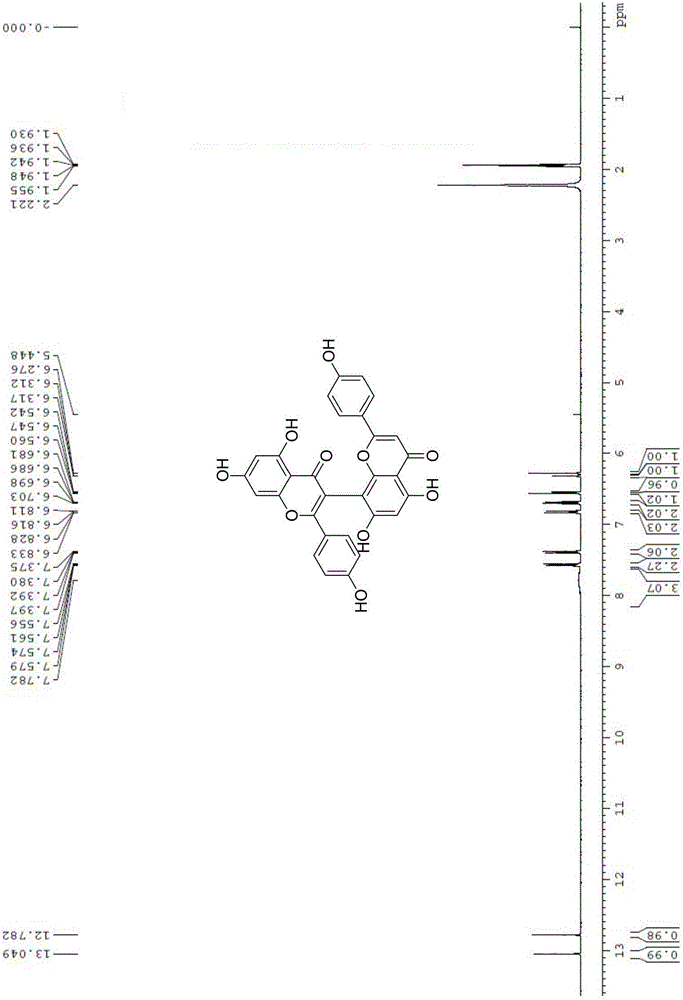
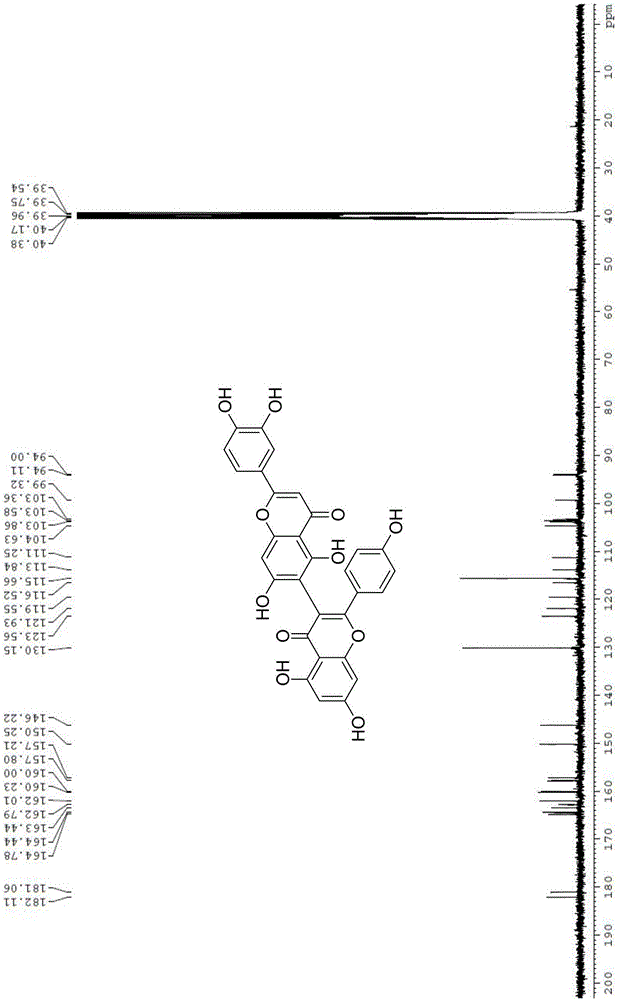
[0023] (1) 5,5',7,7'-tetrahydroxy-2,2'-bis(4-hydroxyphenyl)-4H,4'H-[3,8'-dibenzopyran]-4 ,4'-Diketone
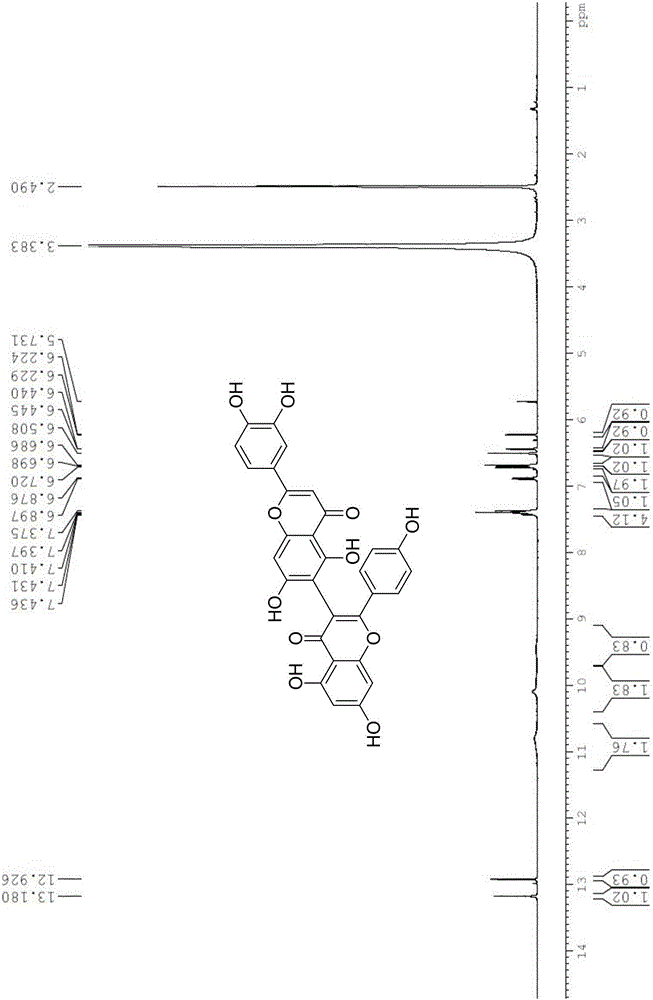
[0024] (2) 2'-(3,4-dihydroxyphenyl)-5,5',7,7'-tetrahydroxy-2-(4-hydroxyphenyl)-4H,4'H-[3,6 '-Dibenzopyran]-4,4'-dione
[0025] Synthetic routes of compounds 1 and 2
[0026]
[0027] Description 1
[0028] 5,5',7,7'-tetrahydroxy-2,2'-bis(4-hydroxyphenyl)-4H,4'H-[3,8'-dibenzopyran]-4,4' - dione
[0029] Add 2.0 g (0.010 mol) of 2-hydroxy-4,6-dimethoxyacetophenone and 2.0 g (0.012 mol) of 4-isopropoxybenzaldehyde into 3 mL of ethanol and stir for 5 min. Then 6ml o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com