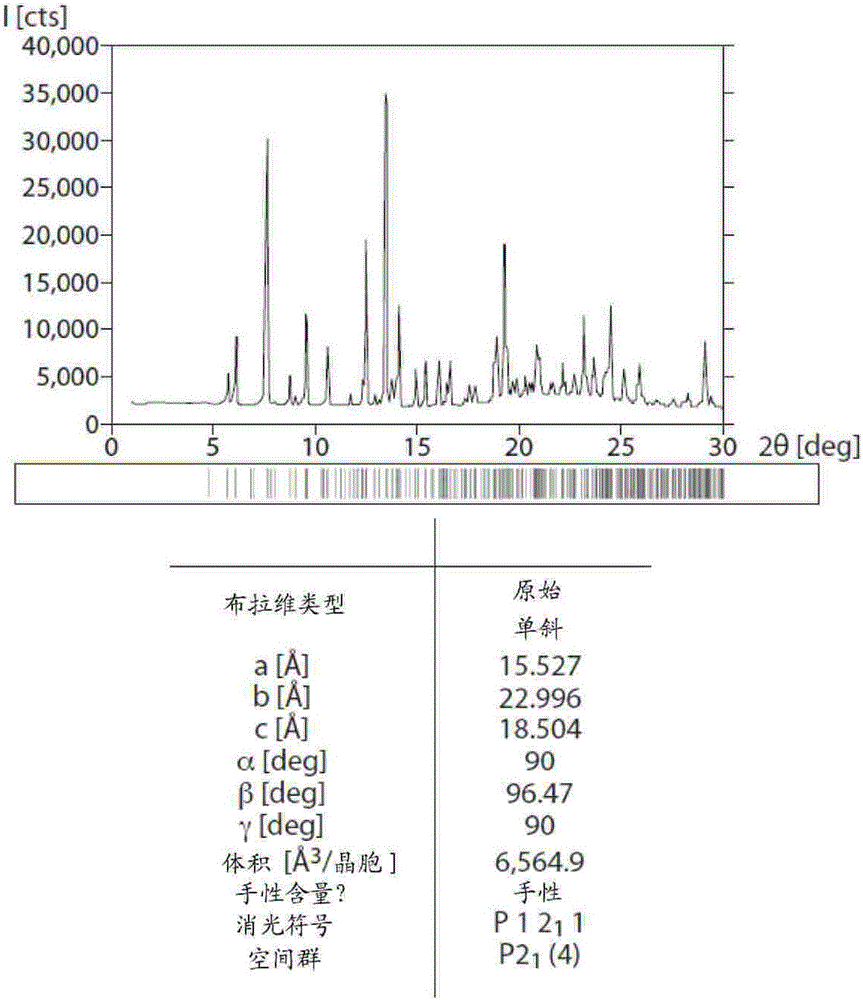
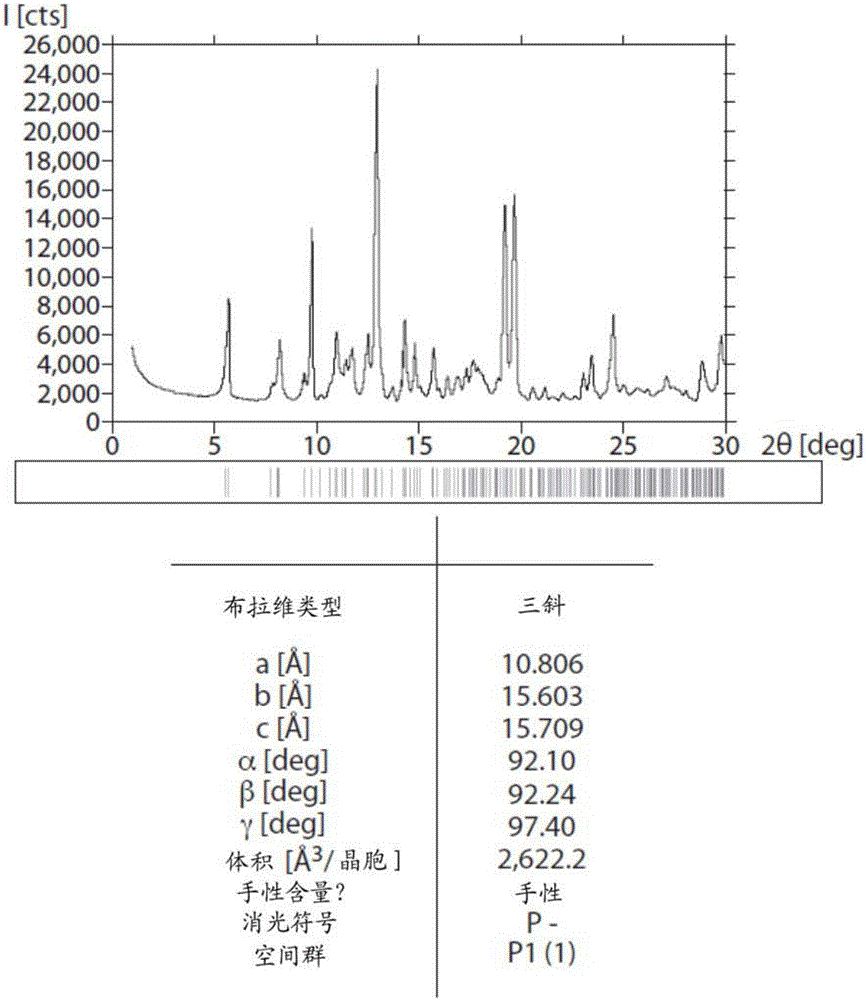
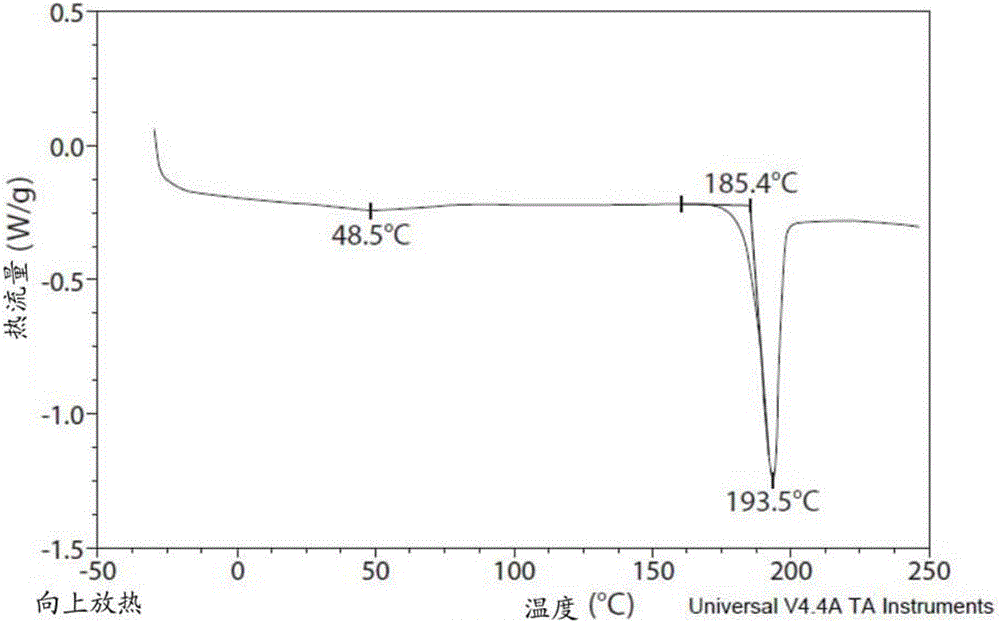
Inhibiting the transient receptor potential A1 ion channel
A C1-C6 compound technology, applied in the field of inhibition of transient receptor potential A1 ion channel, can solve problems such as paroxysmal pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0526] Example 1 (S)-2-(1,3-dimethyl-2,6-dioxo-2,3-dihydro-1H-purin-7(6H)-yl)-N-(2' - (2,2-Dimethylpyrrolidin-1-yl)-[2,5'-bipyrimidine]-4-yl)propionamide
[0527]
[0528] To 2'-(2,2-dimethylpyrrolidin-1-yl)-[2,5'-bipyrimidine]-4-amine (4.2g, 15.5mmol) and (S)-2-( 1,3-Dimethyl-2,6-dioxo-2,3-dihydro-1H-purin-7(6H)-yl)propanoic acid (3.56 g, 14.1 mmol) in DCM (72 mL) To the mixture was added HOAT (1.92 g, 14.1 mmol). The reaction was cooled to 0 °C and pyridine (2.23 g, 28.2 mmol) and DIC (2.67 g, 21.2 mmol) were added. The reaction was warmed to 25-28 °C and stirred overnight. The reaction mixture was quenched with 0.5N HCl. The mixture was added dropwise to n-hexane and the formed precipitate was collected and washed with MeOH to give (S)-2-(1,3-dimethyl-2,6-dioxo-2,3-dihydro-1H -Purin-7(6H)-yl)-N-(2'-(2,2-dimethylpyrrolidin-1-yl)-[2,5'-bipyrimidinyl]-4-yl)propionamide ( 3 g, 19%). 1 H NMR (CDCl 3 )δ9.78(s, 1H), 9.14(s, 2H), 8.54(d, J=5.6Hz, 1H), 7.91(s, 1H), 7....
Embodiment 2
[0529] Example 2 (S)-2-(1,3-dimethyl-2,6-dioxo-2,3-dihydro-1H-purin-7(6H)-yl)-N-(2' - ((S)-2-(trifluoromethyl)pyrrolidin-1-yl)-[2,5'-bipyrimidinyl]-4-yl)propionamide (Compound 2 )
[0530]
[0531] To (S)-2-(1,3-dimethyl-2,6-dioxo-2,3-dihydro-1H-purin-7(6H)-yl)propionic acid (2.44g, 9.67mmol) and (S)-2'-(2-(trifluoromethyl)pyrrolidin-1-yl)-2,5'-bipyrimidin-4-amine (3.3g, 10.6mmol) in DCM (48mL ) was added HOAT (1.3 g, 9.67 mmol). The mixture was cooled to 0 °C. Pyridine (1.5 g, 19.3 mmol) was added dropwise over 30 minutes, followed by DIC (1.8 g, 14.5 mmol). The reaction was stirred at 35 °C for 16 h; it was then diluted with DCM (100 mL). The mixture was saturated with NH 4 Cl (50mL, pre-cooled to 0°C) and brine extraction, with Na 2 SO 4 Dry and concentrate. The residue was purified by chromatography, eluting first with EA:PE (3:2), then DCM:MeOH (30:1 ), then recrystallized from EtOH to give the title compound (4.5 g, 78%), It is a white solid. 1 H NMR (DM...
Embodiment 3
[0533] Example 3 (R)-2-(1,3-dimethyl-2,6-dioxo-2,3-dihydro-1H-purin-7(6H)-yl)-N-(2' - ((S)-2-(Trifluoromethyl)pyrrolidin-1-yl)-[2,5'-bipyrimidinyl]-4-yl)propionamide
[0534]
[0535] The mother liquor from Example 2 after recrystallization in EtOH was concentrated. The residue was loaded for chiral preparative HPLC purification to give (R)-2-(1,3-dimethyl-2,6-dioxo-2,3-dihydro-1H-purine-7 (6H)-yl)-N-(2'-((S)-2-(trifluoromethyl)pyrrolidin-1-yl)-[2,5'-bipyrimidinyl]-4-yl)propionamide , which is a white solid. 1 H NMR (DMSO-d 6 )δ11.48(s, 1H), 9.25(s, 2H), 8.69(d, J=5.6Hz, 1H), 8.33(s, 1H), 7.83(d, J=5.6Hz, 1H), 5.81( q, J=7.2Hz 1H), 5.13(t, 1H), 3.71(m, 2H), 3.69(s, 3H), 3.17(s, 3H), 2.14(m, 4H), 1.88(d, J= 7.2Hz, 3H).MH + 545.de: 99%. *
[0536] *Chiral HPLC method conditions: column: CHIRALPAK IB, 150*4.6mm, 5μm; mobile phase: A: hexane (HPLC grade); B: EtOH (HPLC grade); flow rate: 0.8mL / min; gradient: 30% B kept for 25 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com