Carboline compounds, and synthetic method and anti-acetylcholinesterase activity thereof
A synthesis method and compound technology, which are applied in the fields of carboline compounds and their synthesis, and anti-acetylcholinesterase activity, can solve the problems that have not yet been seen, and achieve the effect of strong inhibitory activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0047] The present invention will be described in detail below in combination with specific embodiments.
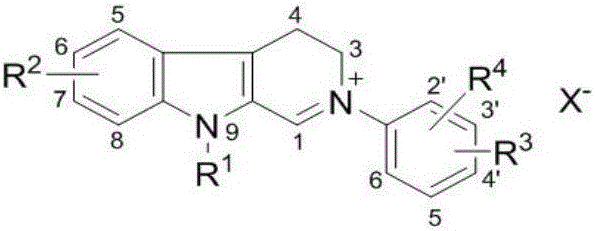
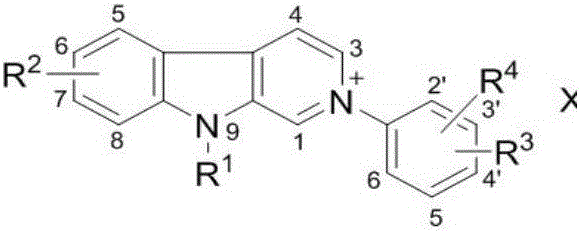
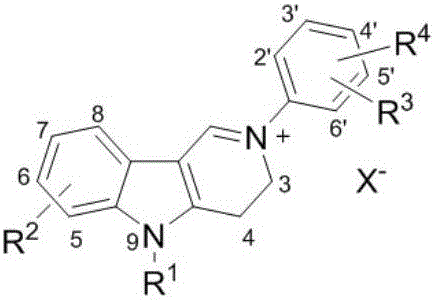
[0048] According to the structural characteristics, the carboline compounds involved in the present invention can be divided into A, B, C, and D types, wherein B and D types are 2-aryl carboline salt compounds, and A and C types are 2-aryl -3,4-Dihydrocarboline salt compound. The activity test shows that the compound involved in the present invention has significant inhibitory activity on AChE and BChE, and has great potential as an active ingredient for preparing a drug for treating AD.
[0049] The carboline compound has the following molecular structure:
[0050] Class A:
[0051]
[0052] Class B:
[0053]
[0054] Class C:
[0055]
[0056] Class D:
[0057]
[0058] in:
[0059] R 1 It is a hydrogen atom, an alkyl group, a cycloalkyl group, an unsaturated hydrocarbon group, an aryl group or an aromatic heterocyclic group, a heterocyclic hydrocarbo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com