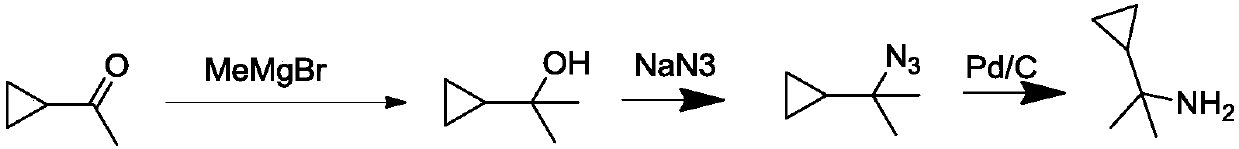
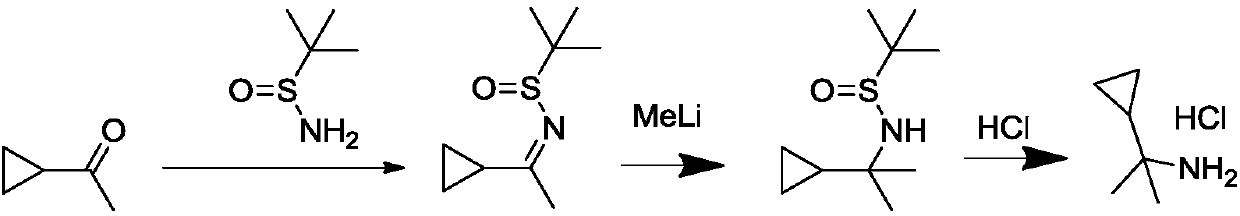
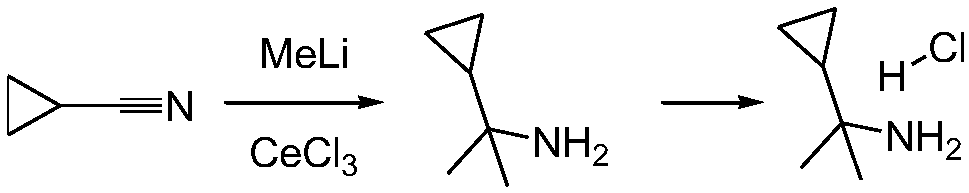
A kind of synthetic method of (1-cyclopropyl-1-methyl) ethylamine hydrochloride
A technique for the synthesis of ethylamine hydrochloride and its synthesis method, which is applied in the field of synthesis of ethylamine hydrochloride, can solve the problems of low yield, unsuitable amplification, and harsh reaction conditions, and achieve high yield, easy operation, and reaction The effect of process stabilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029] Dissolve cerium trichloride (238g, 0.9mol, 3.0eq) in 2L anhydrous THF, stir at room temperature for 2 hours, then cool the reaction solution to -78°C, then add methyllithium in tetrahydrofuran dropwise (methyllithium The concentration is 1mol / L, 1.2L is added dropwise), wherein the addition of methyl lithium is (1.2mol, 4.0eq); after the dropwise addition, the reaction is continued for 1 hour, and then cyclopropylnitrile is added dropwise to the reaction solution (20g, 0.30mol, 1.0eq) THF solution 60ml, after the dropwise addition, continue to react for 1h, then the reaction solution naturally rises to room temperature and reacts for 4h, then adds 100ml of ammonia water to the reaction solution to quench the reaction, filter the reaction solution, filter The cake was washed with DCM, and the filtrate was concentrated to obtain 20 g of crude product (1-cyclopropyl-1-methyl) ethylamine,
[0030] Crude product (1-cyclopropyl-1-methyl) ethyl amine 20g is dissolv...
Embodiment 5
[0035] Dissolve cerium trichloride (238g, 0.9mol, 3.0eq) in 2L anhydrous THF, stir at room temperature for 2 hours, then cool the reaction solution to -78°C, then add methyllithium in tetrahydrofuran dropwise (methyllithium The concentration is 1mol / L, 1.2L is added dropwise), wherein the addition of methyl lithium is (1.2mol, 4.0eq); after the dropwise addition, the reaction is continued for 1 hour, and then cyclopropylnitrile is added dropwise to the reaction solution (20g, 0.30mol, 1.0eq) of THF solution 60ml, after the dropwise addition, continue to react for 4h, then add 100ml of ammonia water to the reaction solution to quench the reaction, filter the reaction solution, wash the filter cake with DCM, concentrate the filtrate to obtain crude Product (1-cyclopropyl-1-methyl) ethylamine 12g.
[0036] Crude product (1-cyclopropyl-1-methyl) ethyl amine 12g is dissolved in the ethyl acetate of 100ml, feeds hydrochloric acid gas to it then to the solid that precipitates no long...
Embodiment 6
[0038] Dissolve cerium trichloride (2380g, 9.6mol, 3.0eq) in 20L of anhydrous THF, stir at room temperature for 2 hours, then cool the reaction solution to -78°C, then add methyllithium tetrahydrofuran solution dropwise (methyllithium The concentration is 1mol / L, dripping 12L), wherein the addition of methyllithium is (12mol, 4.0eq); Continue to react for 2.5 hours after dropping, add dropwise cyclopropyl nitrile (200g, 30mol, 1.0eq) of THF solution 600ml, after the dropwise addition, continue to react for 4h, then the reaction solution naturally rose to room temperature and reacted overnight, then added ammonia water 1000ml to the reaction solution to quench the reaction, stirred for 2 hours, filtered the reaction solution, filtered The cake was washed with DCM and the filtrate was concentrated to give crude product 195g.
[0039] Crude product 195g is dissolved in the ethyl acetate of 100ml, feeds hydrochloric acid gas to it then to the solid that separates out no longer inc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com