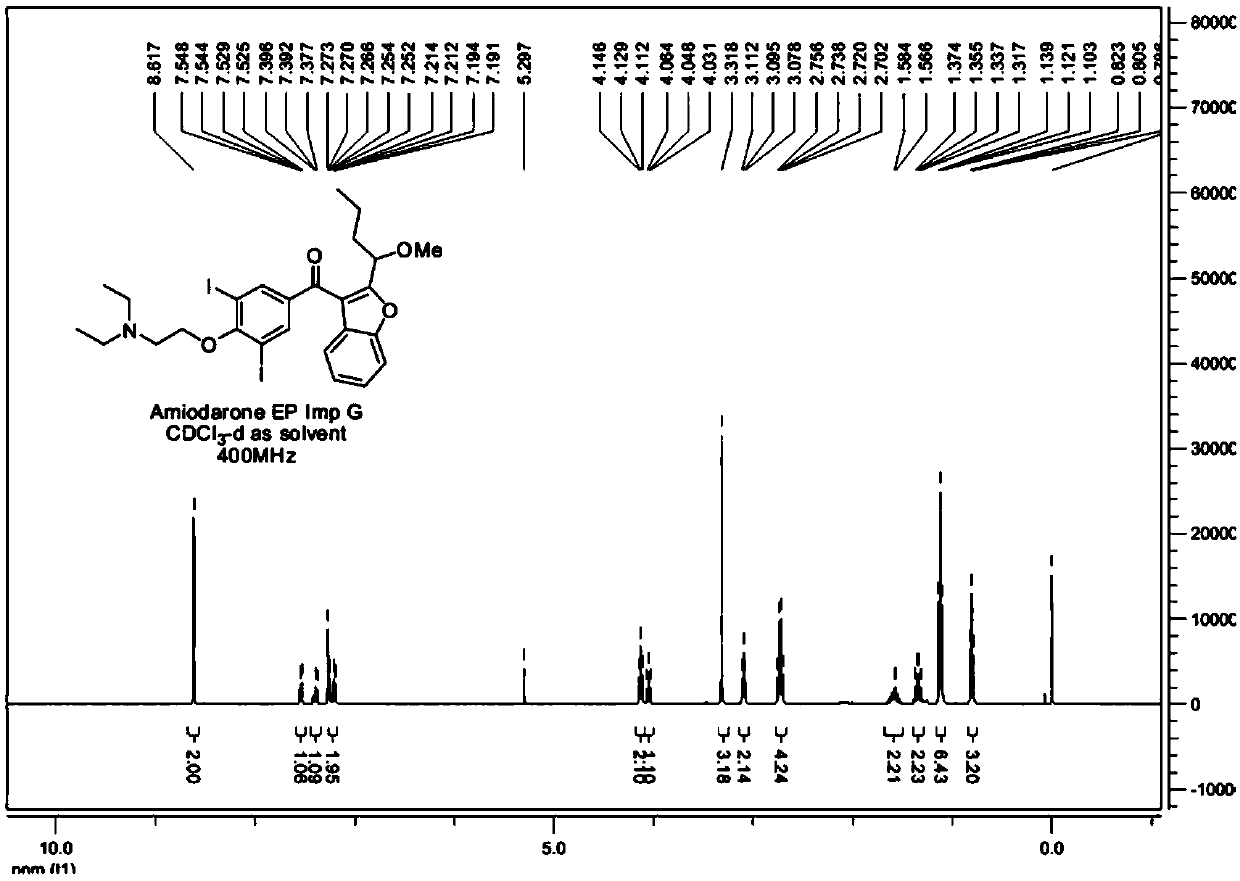
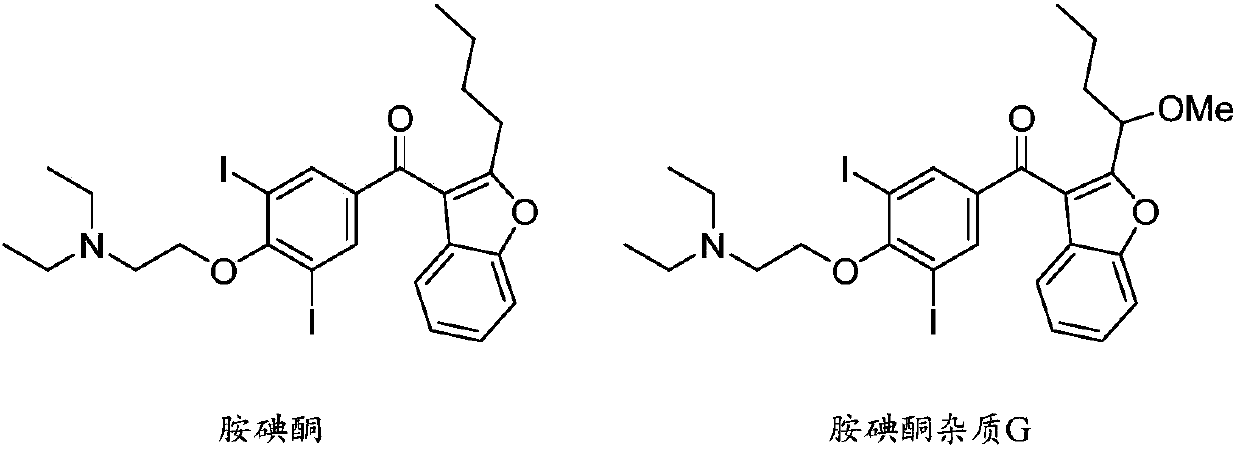
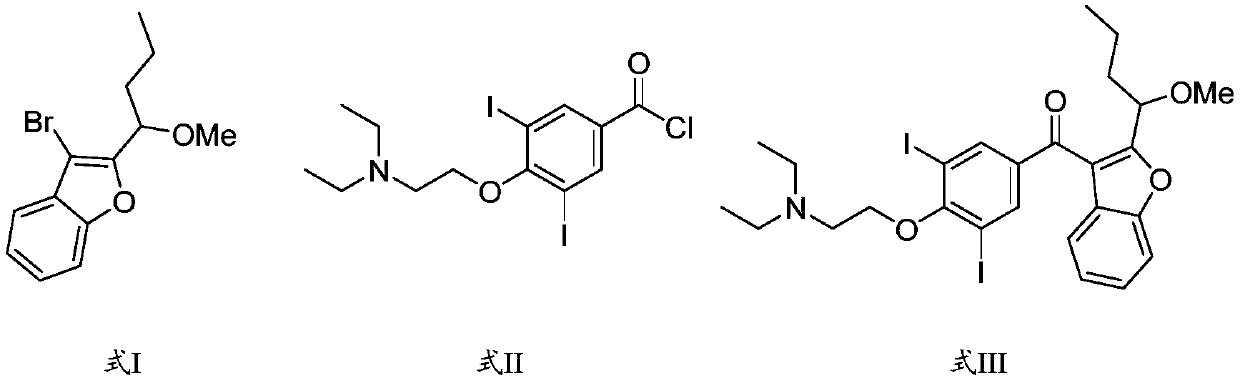
The synthetic method of amiodarone impurity g and the application of amiodarone impurity g
A synthesis method and technology of amiodarone are applied in the synthesis of amiodarone impurity G, and the application field of amiodarone impurity G can solve the problems such as an undisclosed high-efficiency synthesis method of amiodarone impurity G, and achieve high purity and cost. Low, high-yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The invention provides a kind of synthetic method of amiodarone impurity G, comprising the following steps:
[0027] (1) Under the protection of an inert gas, the compound of the structure shown in formula I and n-butyllithium carry out the first reaction in the reaction medium;
[0028] (2) After the first reaction is finished, add the compound with the structure shown in formula II to the reaction liquid of the first reaction, and carry out the second reaction to obtain the compound with the structure shown in formula III, namely the amiodarone impurity G.
[0029]
[0030] Wherein, the compound of structure shown in formula I, chemical name is: 3-bromo-2-(1-methoxybutyl) benzofuran; The compound of structure shown in formula II, chemical name is: 4-(2- (Diethylamino)ethoxy)-3,5-diiodobenzoyl chloride.
[0031] It should be noted that the synthesis method provided by the present invention needs to be carried out under anhydrous conditions.
[0032] In a preferred...
Embodiment 1
[0070] Add 90mL of tetrahydrofuran and 6g of the compound of formula I to a 250mL three-necked flask, protect it under nitrogen, cool to -70°C, add 10mL of 2.5mol / L n-butyllithium solution dropwise, and ensure that the temperature is controlled less than -65°C, react at -70°C for 10 minutes after the addition is complete. Then 3 g of the compound represented by formula II and 0.2 g of anhydrous ferric chloride were added, and then returned to room temperature for 2 h of reaction.
[0071]
[0072] The reaction solution after the reaction was purified, concentrated to dryness under reduced pressure with a rotary evaporator, the pressure was set to 0.09Mpa, and the concentration temperature was 35°C. The concentrated solution was separated and purified by HPLC. The specific parameters of HPLC were set as follows:
[0073] Column: DAC 100-10 C18
[0074] Mobile phase: Phase A, 0.1% formic acid; Phase B, methanol
[0075] Flow rate: 150mL / min
[0076] Detection wavelength: 2...
Embodiment 2
[0082] Add 67mL of tetrahydrofuran and 6g of the compound of formula I to a 250mL three-necked flask, protect it under nitrogen, cool to -70°C, add 10mL of 2.5mol / L n-butyl lithium solution dropwise, and ensure that the temperature is controlled less than -65°C, react at -70°C for 10 minutes after the addition is complete. Then, 2.7 g of the compound represented by formula II and 0.1 g of anhydrous ferric chloride were added, and then returned to room temperature for 2 h of reaction.
[0083] According to the purification method provided in Example 1, the reaction solution was purified by HPLC and concentrated under reduced pressure to finally obtain 2.7 g of a light brown oily substance, namely amiodarone impurity G, with a yield of 75%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com