Method for measuring and calculating dynamic heat capacity of adiabatic reaction calorimetry samples
A technology of adiabatic reaction and samples, applied in the field of chemical safety testing technology and instruments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
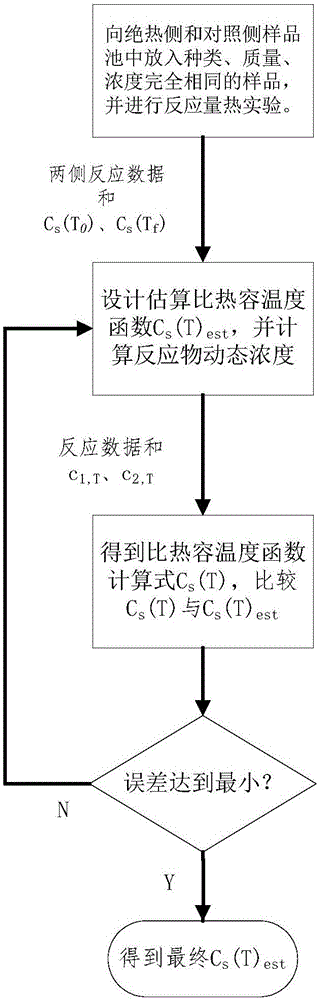
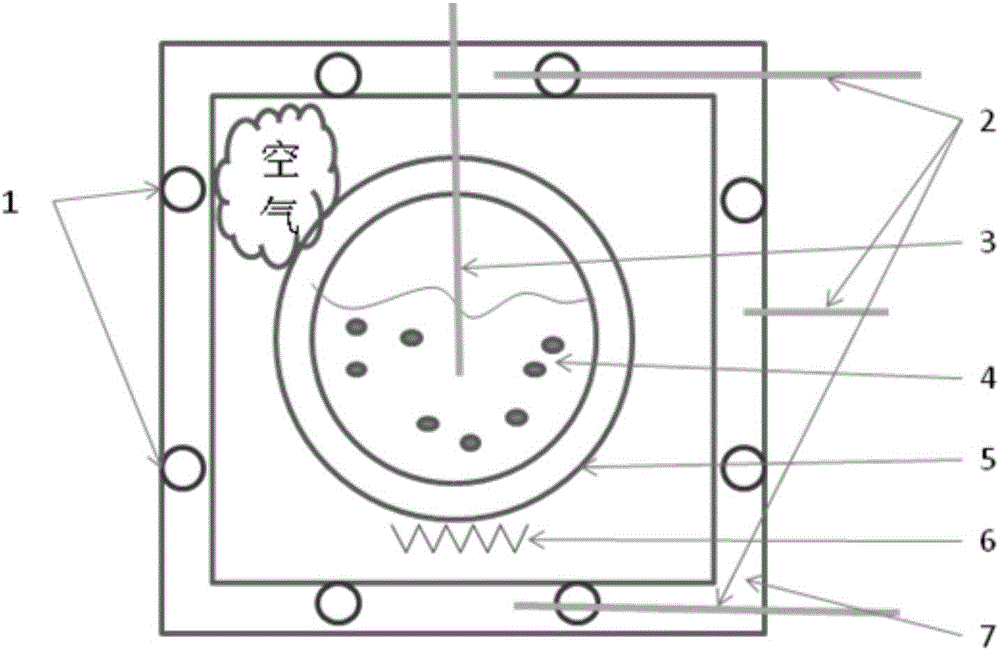
[0044] The present invention will be further described below in conjunction with embodiment and accompanying drawing.
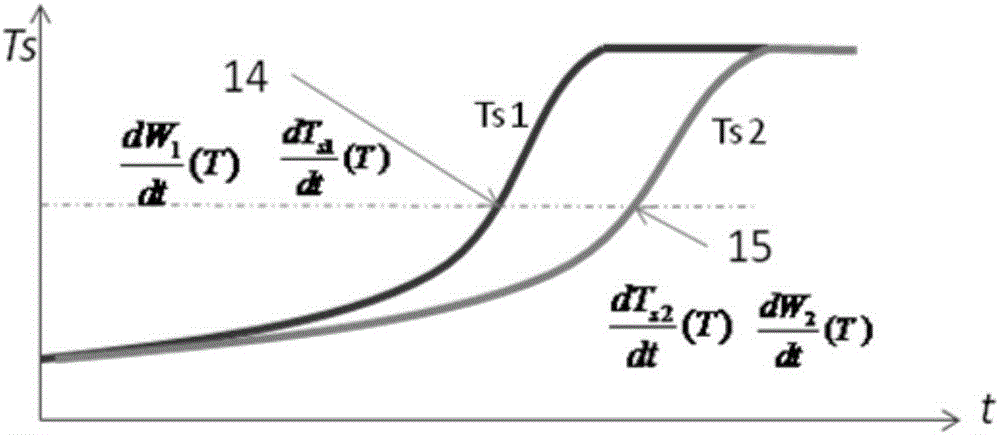
[0045] 1, there is following simplified condition in the method of the present invention: suppose sample specific heat capacity is only a function of temperature:
[0046] In fact, the specific heat capacity of the sample is not only related to temperature during the reaction process, but also affected by the concentration of reactants. For the control side where the reaction path is shifted, because of the difference in reaction progress, the sample concentration at the same temperature is different from that of the adiabatic side, and the actual specific heat capacity-temperature function also deviates from that of the adiabatic side. Compared with the change of specific heat capacity in the whole reaction process, the specific heat capacity deviation is relatively small, so it is ignored in the calculation.
[0047] 2. Before the start of the reaction and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com