Olaparib amorphous compound, and preparation method thereof
A technology of amorphous substances and powders, applied in the direction of organic chemistry, can solve the problems of inability to guarantee drug dissolution stability, low bioavailability, unfavorable absorption, etc., achieve significant industrial application value, easy to achieve large-scale, and solvent residues Reduced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: Preparation of Olaparib Amorphous Form
Embodiment 11
[0037] Under the protection of inert gas, directly heat the raw material of olaparib until it is completely melted; then transfer the molten material to a clean metal container whose temperature is room temperature or pre-cooled to lower than room temperature, so that the material can be rapidly cooled into a solid; The resulting solid was pulverized.
[0038] The obtained powder was taken for X-ray powder diffraction analysis and DSC analysis.
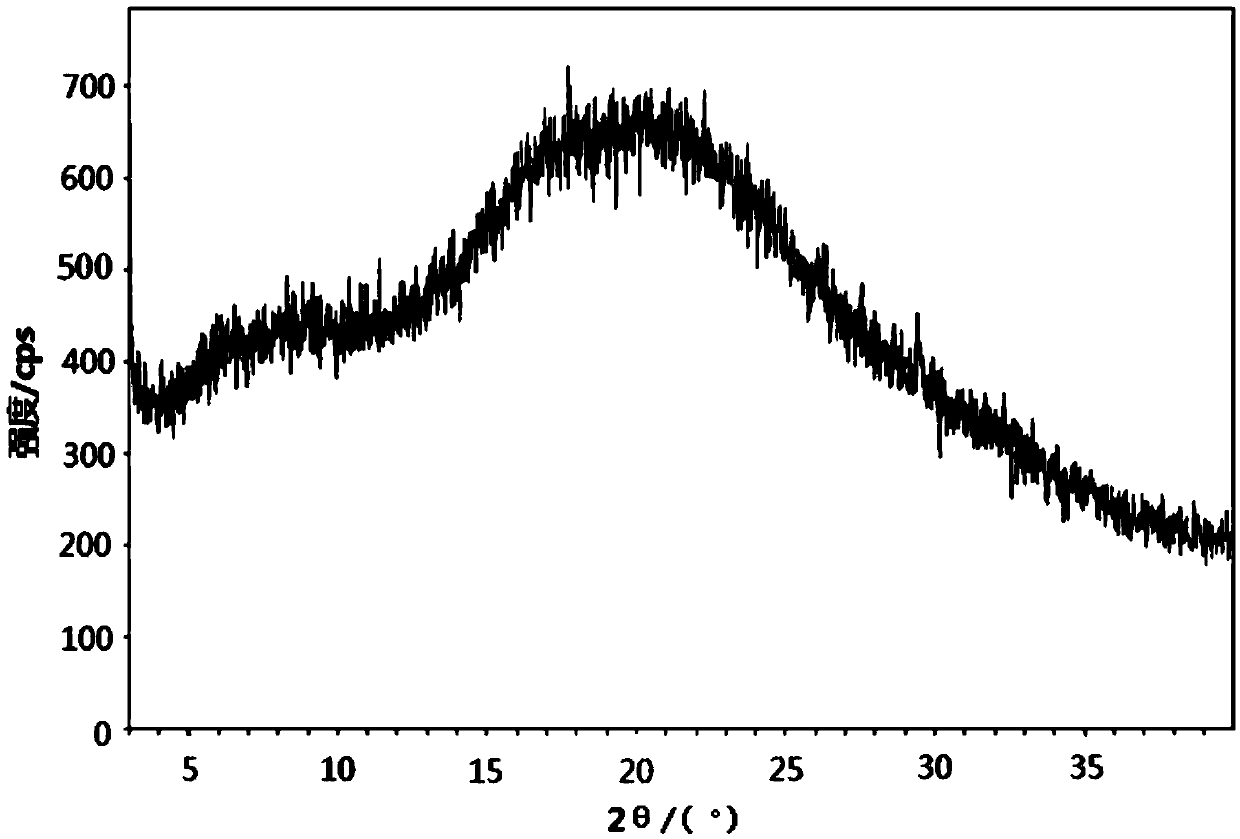
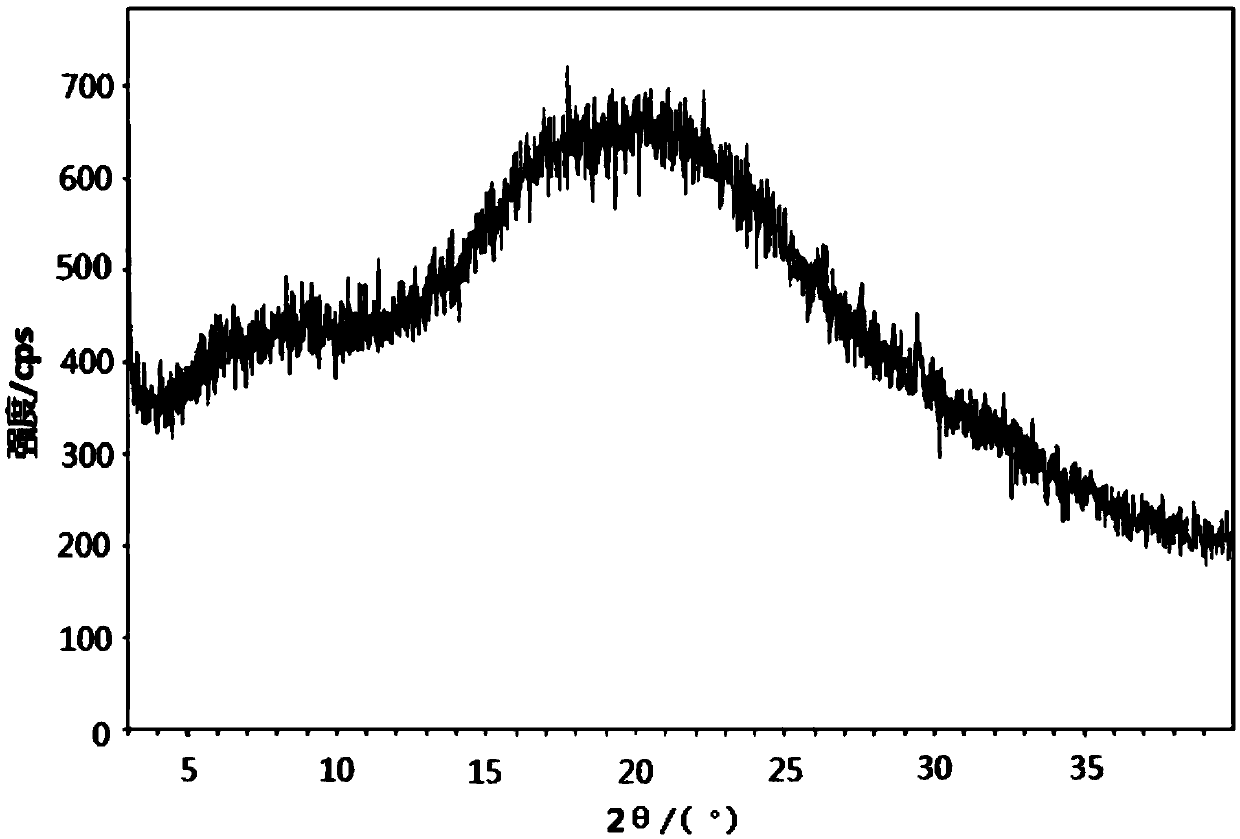
[0039] figure 1 For the XRD spectrum of the obtained powder, by figure 1 It can be seen that the obtained powder is amorphous olaparib, with a broad peak between 12.0 and 40.0 degrees in 2θ and a shoulder peak between 5.0 and 15.0 degrees in 2θ.
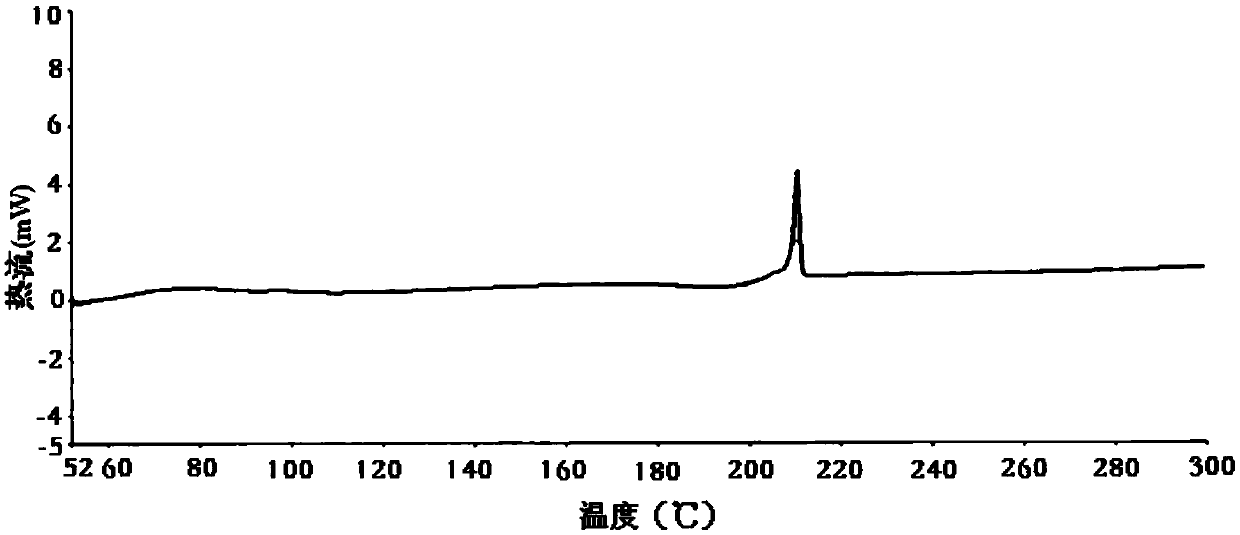
[0040] figure 2 For the DSC spectrum of the obtained powder, by figure 2 It can be seen that the obtained powder undergoes exothermic transformation at about 210.6°C.
Embodiment 12
[0042] Under the protection of an inert gas, directly heat the raw material of olaparib until it is completely melted; then pour the molten material into a stirred ice-water mixture to rapidly cool the material into shape; filter, collect the solid powder, and vacuum-dry at 55°C .
[0043] Through X-ray powder diffraction analysis and DSC analysis, the obtained powder is the described amorphous olaparib.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap