Adenoviral vector-based vaccine against enterovirus infection
An enterovirus and recombinant adenovirus technology, applied in the field of immunity, can solve the problems of inducing immunogenicity and obstruction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
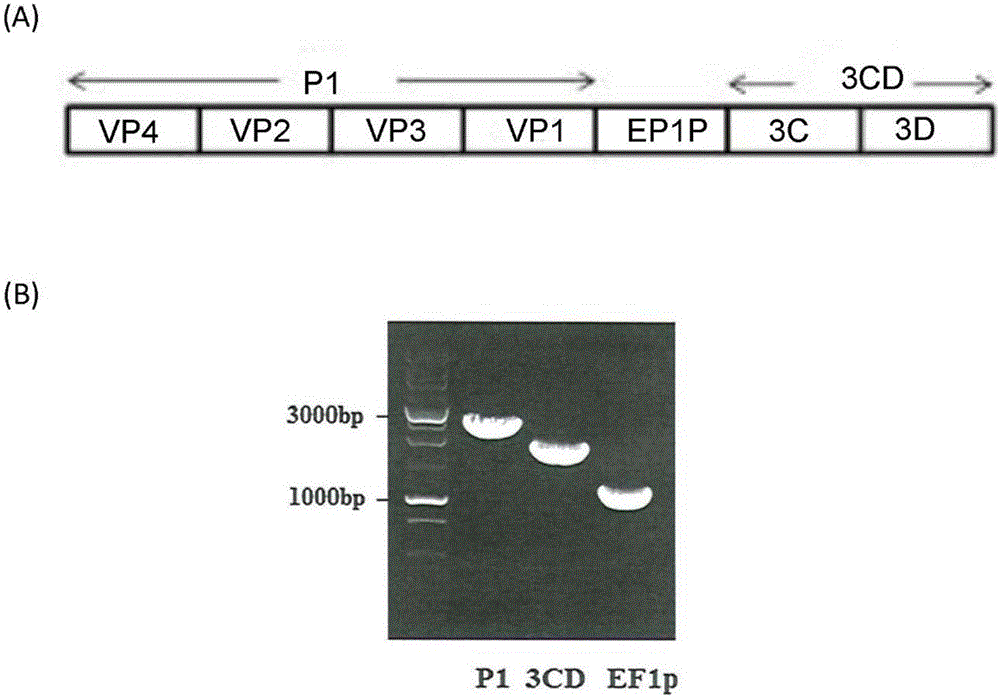
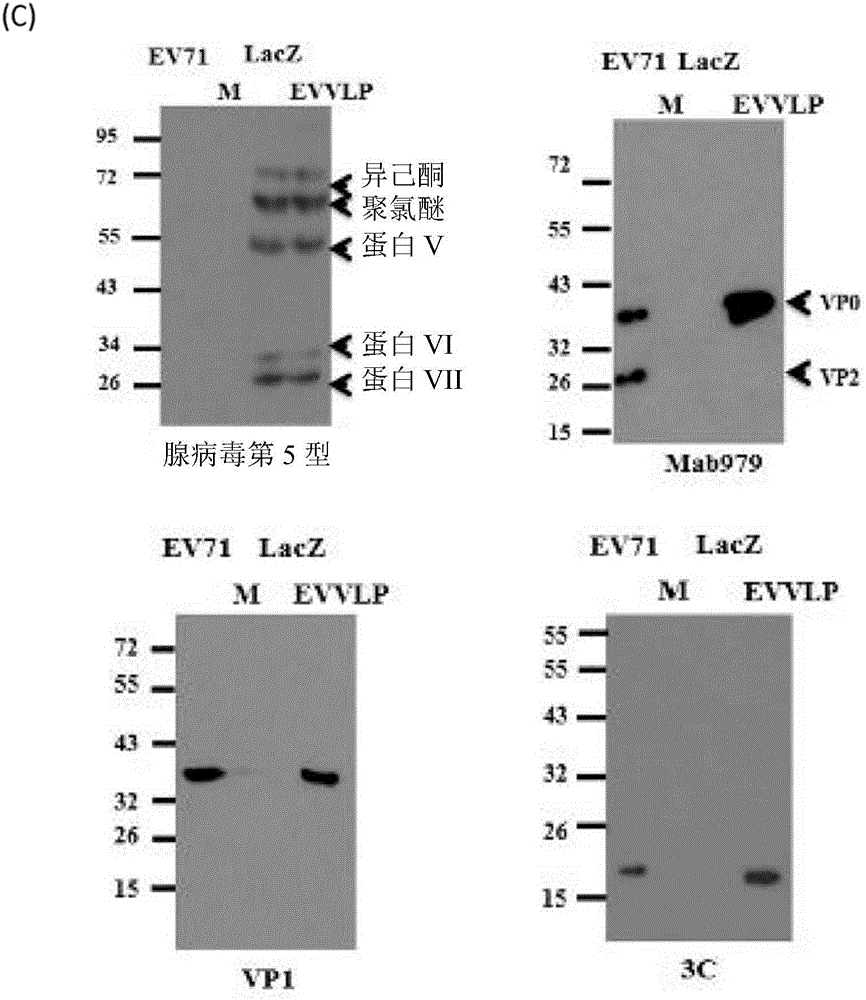
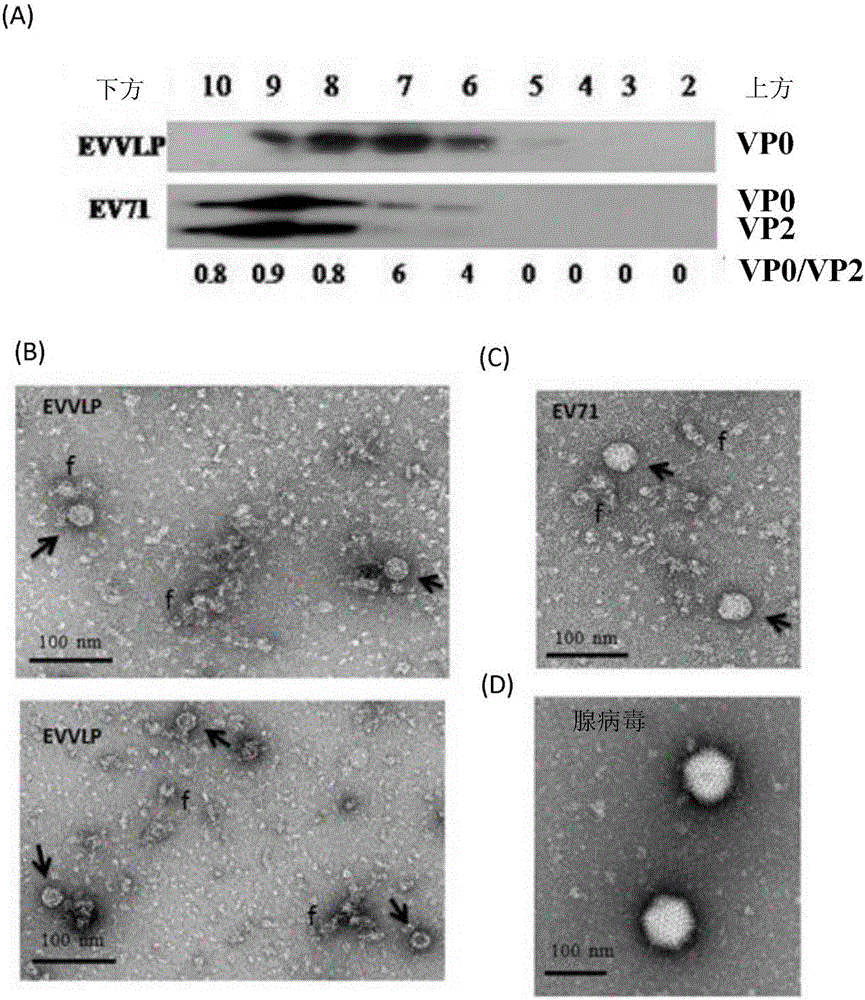
[0082] In this study, we have designed and genetically engineered a recombinant adenoviral vector, Ad-EVVLP, carrying EV71P1 and 3CD genes, which were inserted into the E1 / E3 deleted adenoviral genome. Ad-EVVLP can be produced in HEK-293A cells. In addition to Ad-EVVLP particles, virus-like particles (VLP) are formed by physically linking with EV71-type capsid proteins VPO, VP1 and VP3; wherein these capsid proteins are the products of the P1 gene. The virus particles were cleaved by 3CD protease and confirmed to be produced by Ad-EVVLP-producing cells, and identified by transmission electron microscopy and western blotting. Immunogenicity studies in mice showed that Ad-EVVLP-immunized antisera can neutralize EV71 type B4 and C2 genotypes. VLP-specific CD4 + and CD8 + Activation of / IFN-γ T cells is associated with the induction of Th1 / Th2-balanced IFN-, IL-17, IL-4, and IL-13; in contrast, FI-EV71 only induces Th2-mediated neutralization Antibody against EV71 and low VLP-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com