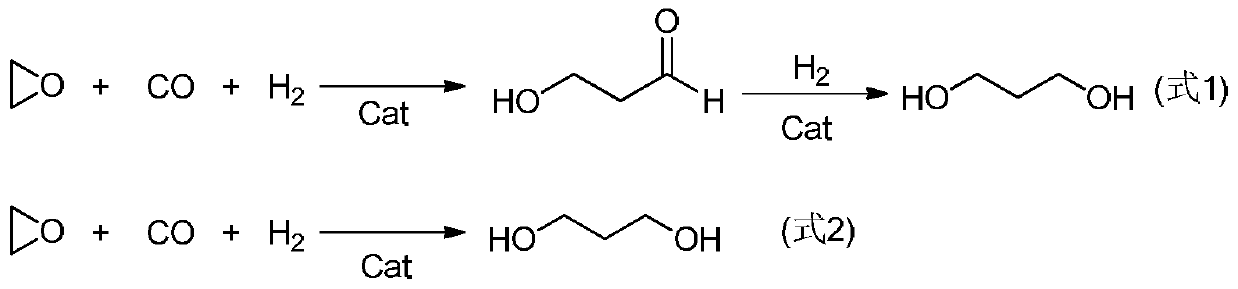
The synthetic method of 3-hydroxy propionaldehyde
A technology of hydroxypropionaldehyde and its synthesis method, which is applied in chemical instruments and methods, preparation of heterocyclic compounds, organic compound/hydride/coordination complex catalyst, etc., can solve the problems of low yield of 3-hydroxypropionaldehyde, etc. Achieve strong electron donating ability and increase yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
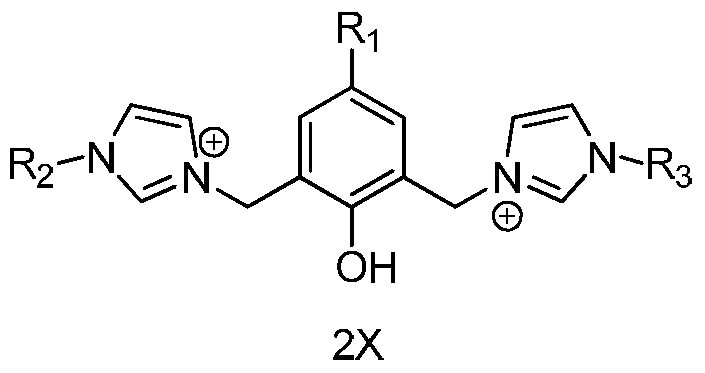
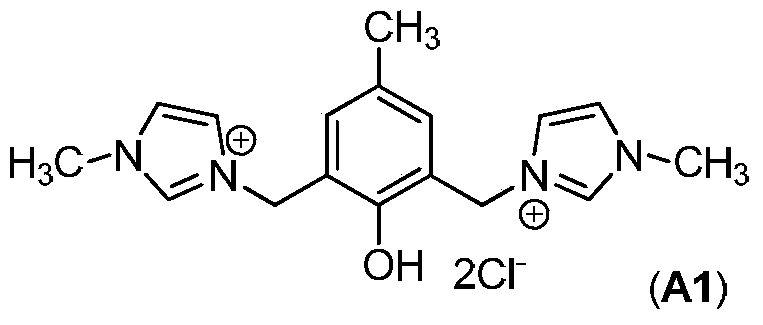
[0035] Add 2050mg (10mmol) 2,6-dichloromethyl-p-cresol, 3280mg (40mmol) 1-methylimidazole, 40mL 1,4-dioxane into a 100mL reaction tube, and replace the air in the reaction tube with nitrogen three times. Under nitrogen atmosphere, react at 100°C for 24 hours. After the reaction was completed, the resulting precipitate was filtered, washed with 1,4-dioxane and diethyl ether, and vacuum-dried to obtain 2285 mg of light yellow solid, which was the N-heterocyclic carbene ligand precursor A1.
[0036]
[0037] Take 1 mmol of N-heterocyclic carbene ligand precursor A1, 1 mmol of cobalt carbonate, and 30 mL of methyl tert-butyl ether, and add them into a 100 mL reaction kettle. High-purity nitrogen and syngas (V H2 / V CO =1 / 1) replace the gas in the kettle three times, heat to 65° C., and stir for 4 hours. Add the oxirane of 50mmol; Add synthesis gas (V H2 / V CO =2 / 1), make the system pressure 12MPa; react at 100°C for 3 hours. After fully cooling the reactor body to 0°C, th...
Embodiment 2
[0039] Add 2940mg (10mmol) 2,6-dibromomethyl-p-cresol, 3280mg (40mmol) 1-methylimidazole, 40mL 1,4-dioxane into a 100mL reaction tube, replace the air in the reaction tube with nitrogen three times, and Under nitrogen atmosphere, react at 100° C. for 20 hours. After the reaction was completed, the resulting precipitate was filtered, washed with 1,4-dioxane and diethyl ether, and vacuum-dried to obtain 2980 mg of light yellow solid, which was the N-heterocyclic carbene ligand precursor A2.
[0040]
[0041] Take 1 mmol of N-heterocyclic carbene ligand precursor A2, 1 mmol of cobalt carbonate, and 30 mL of methyl tert-butyl ether, and add them into a 100 mL reaction kettle. High-purity nitrogen and syngas (V H2 / V CO =1 / 1) replace the gas in the kettle three times, heat to 65° C., and stir for 4 hours. Add the oxirane of 50mmol; Add synthesis gas (V H2 / V CO =2 / 1), make the system pressure 12MPa; react at 100°C for 3 hours. After fully cooling the reactor body to 0°C, t...
Embodiment 3
[0043] Add 3880mg (10mmol) 2,6-diiodomethyl-p-cresol, 3280mg (40mmol) 1-methylimidazole, 50mL 1,4-dioxane into a 100mL reaction tube, and replace the air in the reaction tube with nitrogen three times. Under nitrogen atmosphere, react at 100°C for 24 hours. After the reaction was completed, the resulting precipitate was filtered, washed with 1,4-dioxane and diethyl ether, and vacuum-dried to obtain 3870 mg of light yellow solid, which was the N-heterocyclic carbene ligand precursor A3.
[0044]
[0045] Take 1 mmol of N-heterocyclic carbene ligand precursor A3, 1 mmol of cobalt carbonate, and 30 mL of methyl tert-butyl ether, and add them into a 100 mL reaction kettle. High-purity nitrogen and syngas (V H2 / V CO =1 / 1) replace the gas in the kettle three times, heat to 65° C., and stir for 4 hours. Add the oxirane of 50mmol; Add synthesis gas (V H2 / V CO =2 / 1), make the system pressure 12MPa; react at 100°C for 3 hours. After fully cooling the reactor body to 0°C, the pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com