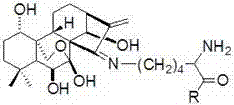
Oridonin Schiff base derivative and preparation method and uses thereof
A technology of oridonin A and Schiff base, applied in the field of medicine, can solve the problems of poor anti-tumor effect, low blood drug concentration, toxic and side effects, etc.
- Summary
- Abstract
- Description
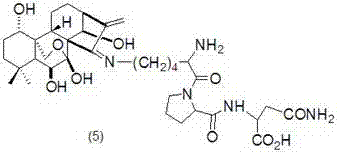
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of Oridonine A Lysine Schiff Base (1)
[0025] The structure of compound (1) is shown in the following formula:
[0026]
[0027] Take 546mg (1mmol) L-Fmoc-Lys (Dde)-OCH 3 Add 10 mL of CH containing 2% hydrazine hydrate 2 Cl 2 , stirred for 30 minutes, washed with saturated brine, added equimolar oridonin, refluxed for 12 hours, cooled, filtered, and concentrated to obtain a solid with a yield of 87%.
[0028] The obtained solid was added to 20% piperidine DMF solution, stirred at room temperature for 3 hours, then added 2mol / L NaOH solution for hydrolysis, adjusted to pH 5-7 with dilute hydrochloric acid, evaporated to dryness under reduced pressure, and purified to obtain compound (1) with a yield of 78%. MS (ESI) m / z:537.3 [M] + .
Embodiment 2
[0030] Preparation of Oridonine A Lysine-Alanine Schiff Base (2)
[0031] The structure of compound (2) is shown in the following formula:
[0032]
[0033] Referring to Example 1, get 717mg (1mmol) L-Fmoc-Lys (Dde)-Ala-OCH 3 Instead of 546mg (1mmol) L-Fmoc-Lys (Dde)-OCH 3 , Compound (2) was obtained in a yield of 76%. MS (ESI) m / z:608.4 [M] + .
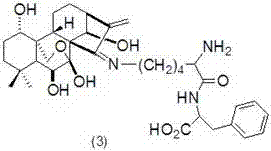
Embodiment 3
[0035] Preparation of Oridonine A Lysine-Phenylalanine Schiff Base (3)
[0036] The structure of compound (3) is shown in the following formula:
[0037]
[0038] Referring to Example 1, get 793mg (1mmol) L-Fmoc-Lys (Dde)-Phe-OCH 3 Instead of 546mg (1mmol) L-Fmoc-Lys (Dde)-OCH 3 , Compound (3) was obtained in a yield of 77%. MS (ESI) m / z:684.4 [M] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com