Biflavone-iron complex and its preparation method and application
A technology of iron complexes and bisflavonoids, which is applied to iron organic compounds, iron group organic compounds without C-metal bonds, pharmaceutical formulations, etc., can solve problems such as unreported research on the synthetic biological activity of bisflavonoid complexes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
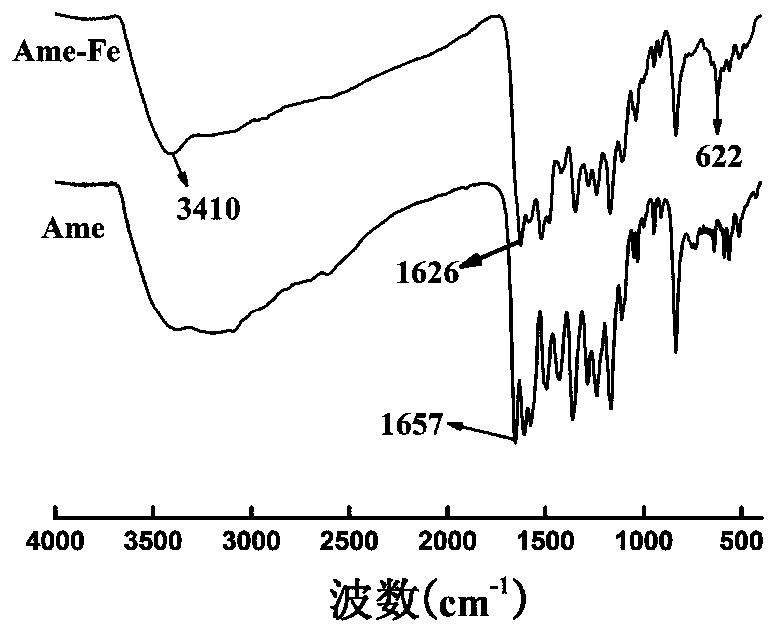
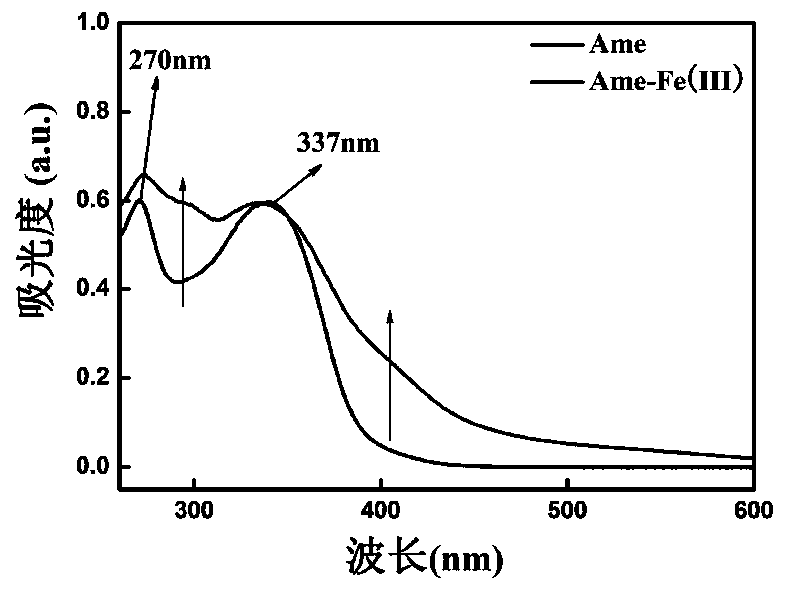
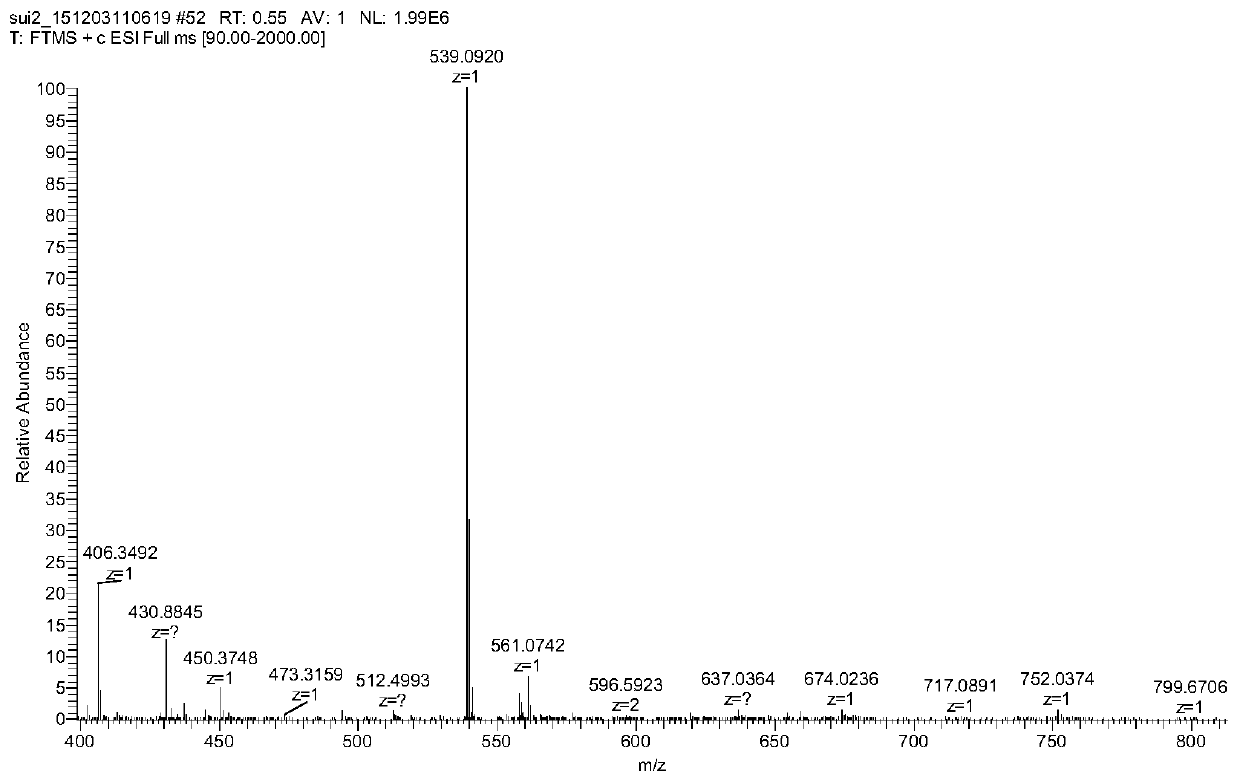
[0039] Accurately weigh 53.8mg of Ame in a round bottom flask, dissolve it with 5mL of ethanol, accurately weigh 20.2mg of ferric nitrate nonahydrate, dissolve it with 5mL of ethanol, add the ferric nitrate solution to the Ame solution dropwise, and add ethanol dropwise to the reaction solution -Ammonia water (V / V, 3:1) solution, adjust the pH to 6, keep at 30°C for 4-5h, precipitate, filter, wash with ethanol and water in turn, recrystallize in DMSO, and freeze-dry to obtain the Ame-Fe complex .
Embodiment 2
[0041] Accurately weigh 53.8 mg of Ame in a round bottom flask, dissolve it with 5 mL of 90% ethanol, accurately weigh 20.2 mg of ferric nitrate nonahydrate, dissolve it with 5 mL of 90% ethanol, add the ferric nitrate solution dropwise to the Ame solution, and add to the reaction solution Add ethanol-sodium ethoxide solution dropwise to adjust the pH to 5, keep at 30°C for 4-5h, precipitate, filter, wash with ethanol and water successively, recrystallize in DMSO, and freeze-dry to obtain the Ame-Fe complex.
Embodiment 3
[0043] Accurately weigh 53.8mg of Ame in a round bottom flask, dissolve it with 5mL of methanol, accurately weigh 20.2mg of ferric nitrate nonahydrate, dissolve it with 5mL of methanol, add the ferric nitrate solution to the Ame solution dropwise, and add methanol dropwise to the reaction solution - Sodium methoxide solution, adjust the pH to 7, keep at 40°C for 3-4 hours to react, a precipitate occurs, filter, wash with methanol and water successively, recrystallize in DMSO, and freeze-dry to obtain the Ame-Fe complex.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com