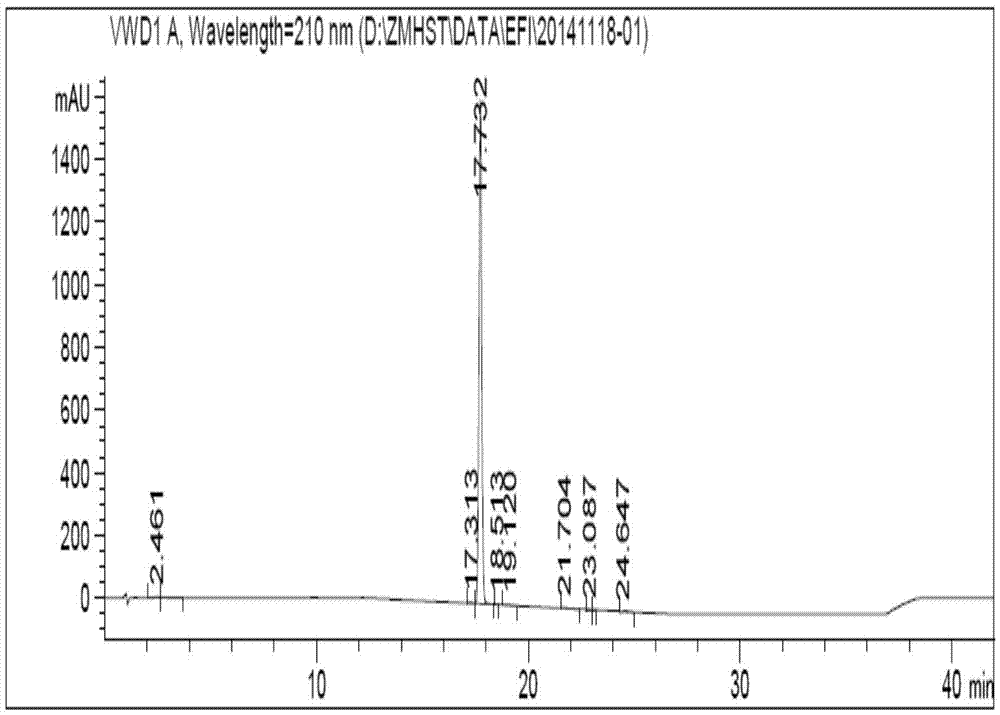
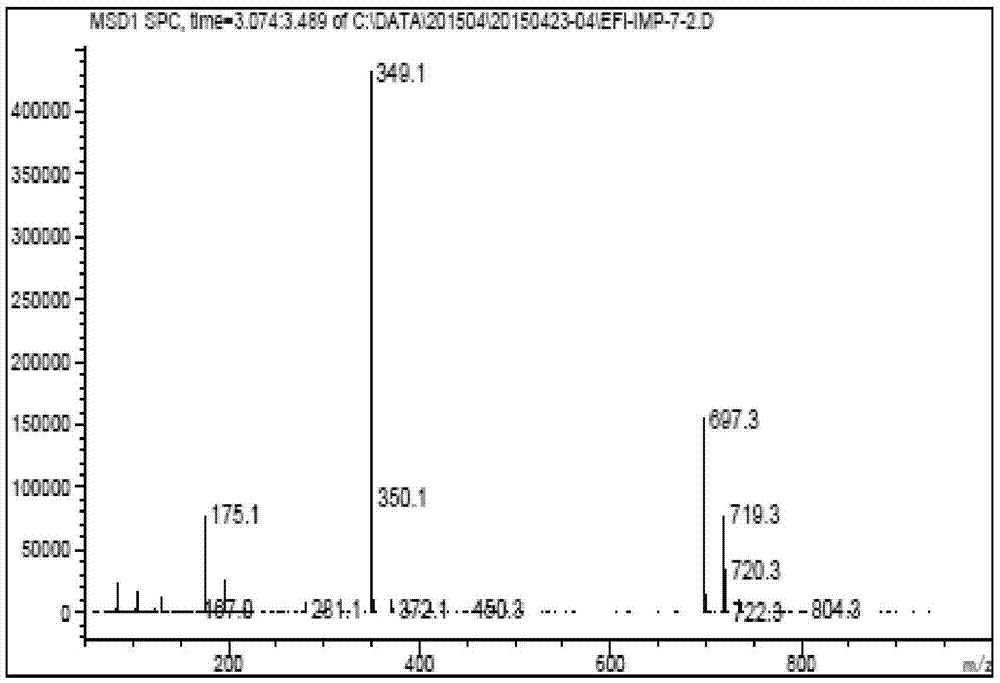
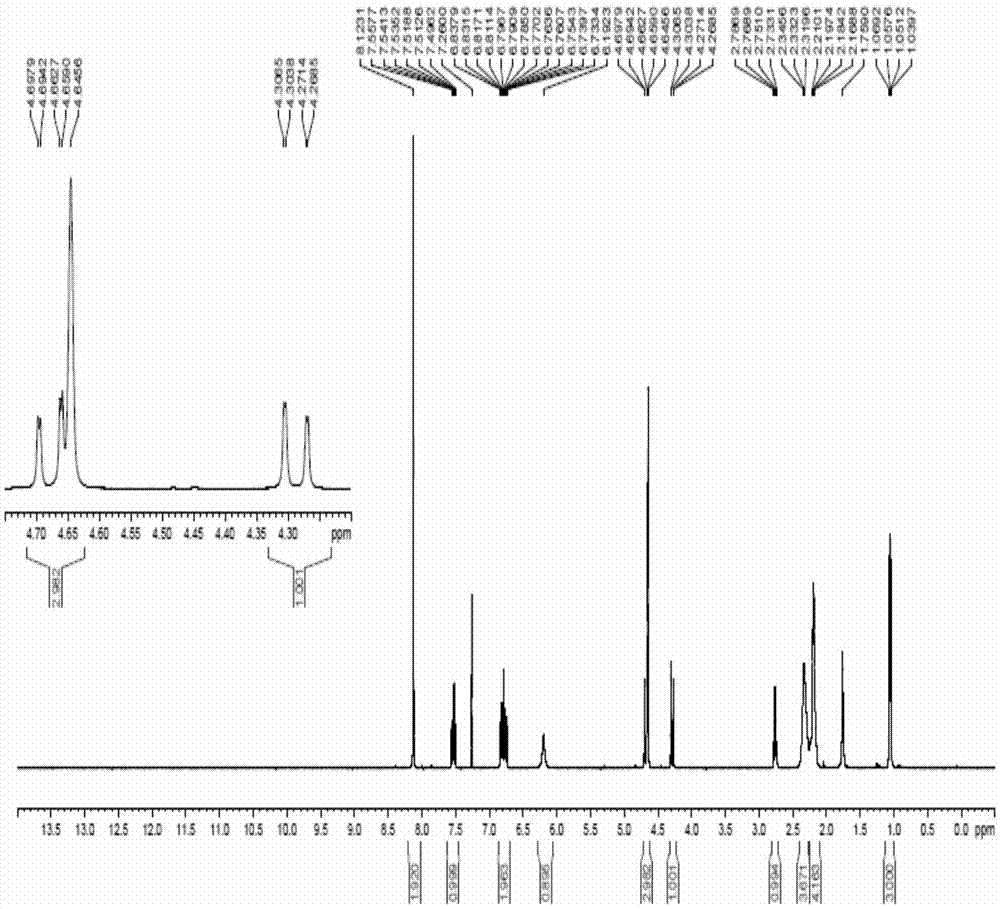
Compound and preparation method and application thereof
A technology of compound and cyclization reaction, which is applied in the field of biomedicine to achieve the effect of mild conditions, simple reaction operation process, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation of embodiment 1 compound 3
[0045] Add 3.4g NaH (60%) into 400ml DMSO, then add 19.6g trimethyl sulfoxide iodide, stir at room temperature for 1 hour, until no bubbles are generated, with exothermic phenomena during the process, add 20.0g compound 2, and stir at room temperature Stir for one hour, take a sample, and TLC monitoring shows that after the raw material has reacted, add 200 ml of water to dilute, then extract with 1000 ml of ethyl acetate, wash the organic phase with 500 ml of water, wash the organic phase with 500 ml of saturated brine and dry it , concentrated to obtain 19.6g of compound 3 with a yield of 93.2%, which was directly used in the next step.
Embodiment 2
[0046] The preparation of embodiment 2 compound 4
[0047] Add 19g of compound 3 obtained in Example 1 to 190 ml of methanol, then add 285 ml of ammonia water, heat to 60°C and continue the reaction, take a sample after 3 hours, monitor the reaction by thin-layer chromatography, the raw material disappears, and the reaction solution is heated at 40°C Concentrate to remove methanol, add 500 ml of ethyl acetate to extract, wash with water, wash with saturated brine, dry and concentrate to obtain 13.1 g of compound 4 with a yield of 65.0% and a purity of 93.2%.
Embodiment 3
[0048] The preparation of embodiment 3 compound 4
[0049] In addition, compound 4 can also be obtained by the following method: add 5.0 g of compound 3 obtained in Example 1 to 25 ml of methanol, then add 50 ml of ammonia water, heat to 50 ° C and continue the reaction, take a sample after 3 hours, and monitor it by thin layer chromatography After the reaction, the raw materials disappeared. The reaction liquid was concentrated at 40°C to remove methanol, and 200 ml of ethyl acetate was added for extraction, washed with water, washed with saturated brine, dried and concentrated to obtain 2.38 g of compound 4 with a yield of 45.0% and a purity of 92.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com