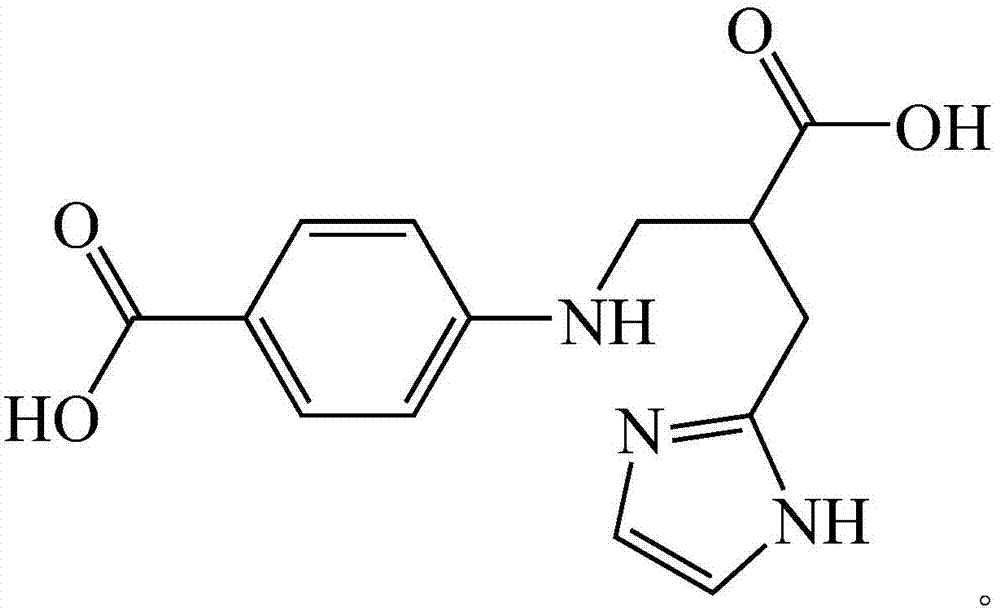
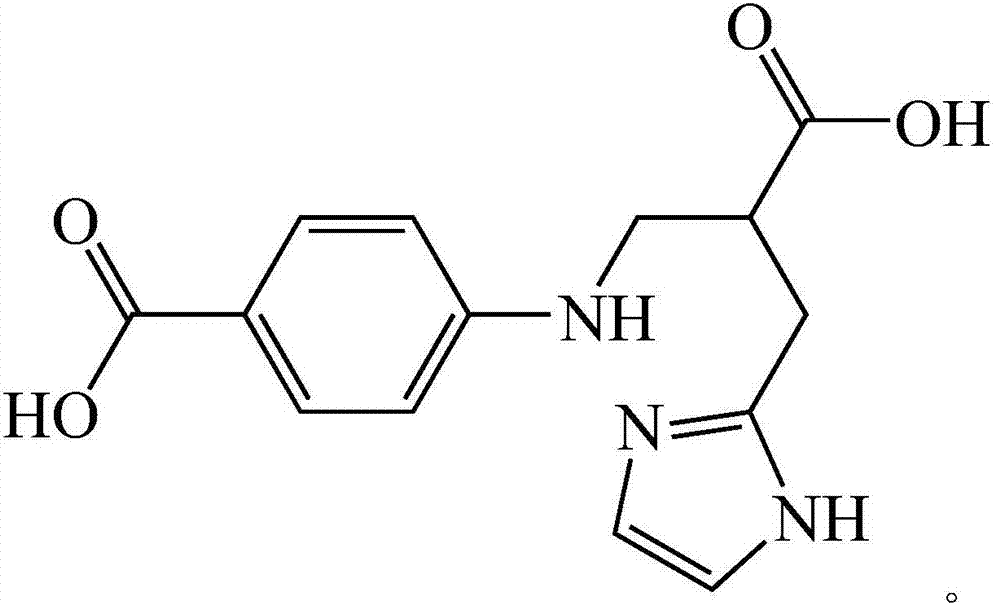
Histidine derived ligand compound and preparation method thereof and application
A technology for ligand compounds and histidine, which is applied in the field of histidine-derived ligand compounds and its preparation, and can solve problems such as hindering the extension of coordination structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Preparation method of histidine derivative ligand compound:
[0026] 1) Weigh 0.32g of solid sodium hydroxide in a flask, measure 3mL of distilled water, and stir until completely dissolved; weigh 1.1g of L-histidine and add it to a three-necked flask, and stir with slight heat until all amino acids are dissolved to obtain a light yellow solution. Weigh 0.7g of benzoic acid-4-formaldehyde and dissolve it in 3mL of ethanol to obtain a colorless solution, add it into a three-neck flask, stir and react at room temperature for 20min to obtain a yellow solution; 2) mix the yellow solution obtained in step 1) for 5min, and divide Add a total of 0.3 g of sodium borohydride solid twice, stir and react in an ice bath for 30 min, until the yellow color of the solution fades completely to obtain a colorless solution; 3) transfer the colorless solution obtained in step 2) to a 50 mL beaker, and evaporate part of the solvent to A large number of white crystals appear, add about ...
Embodiment 2
[0035] (1) Preparation method of histidine-derived ligand compound: 1) Weigh 0.36g of sodium hydroxide solid in a flask, measure 6mL of distilled water, and stir until completely dissolved; weigh 1.4g of L-histidine and add it to a three-necked flask , stirred with slight heat until all the amino acids were dissolved to obtain a light yellow solution, weighed 0.9g of benzoic acid-4-formaldehyde and dissolved it in 6mL of ethanol to obtain a colorless solution, and added it to a three-necked flask, stirred and reacted at room temperature for 40min to obtain a yellow solution; 2) Add the yellow solution obtained in step 1) for about 5 minutes, add 0.35 g of sodium borohydride solid in two times under stirring, stir and react in an ice bath for 30 minutes, until the yellow color of the solution fades completely to obtain a colorless solution; 3) Add the obtained solution in step 2) The colorless solution was transferred to a 50mL beaker, and part of the solvent was evaporated over...
Embodiment 3
[0040] (1) Preparation method of histidine-derived ligand compound: 1) Weigh 0.34g of sodium hydroxide solid in a flask, measure 4mL of distilled water, and stir until completely dissolved; weigh 1.2g of L-histidine and add it to a three-necked flask In medium heat, stir until the amino acid is completely dissolved, and a light yellow solution is obtained. Weigh 0.8g of benzoic acid-4-formaldehyde and dissolve it in 5mL of ethanol to obtain a colorless solution, add it into a three-necked flask, stir and react at room temperature for 30min to obtain a yellow solution; 2) mix the yellow solution obtained in step 1) for 6min, and divide Add a total of 0.32 g of sodium borohydride solid twice, and stir in an ice bath for 35 minutes until the yellow color of the solution fades completely to obtain a colorless solution; 3) Transfer the colorless solution obtained in step 2) to a 50 mL beaker, and evaporate part of the solvent to A large number of white crystals appeared. Add about...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com