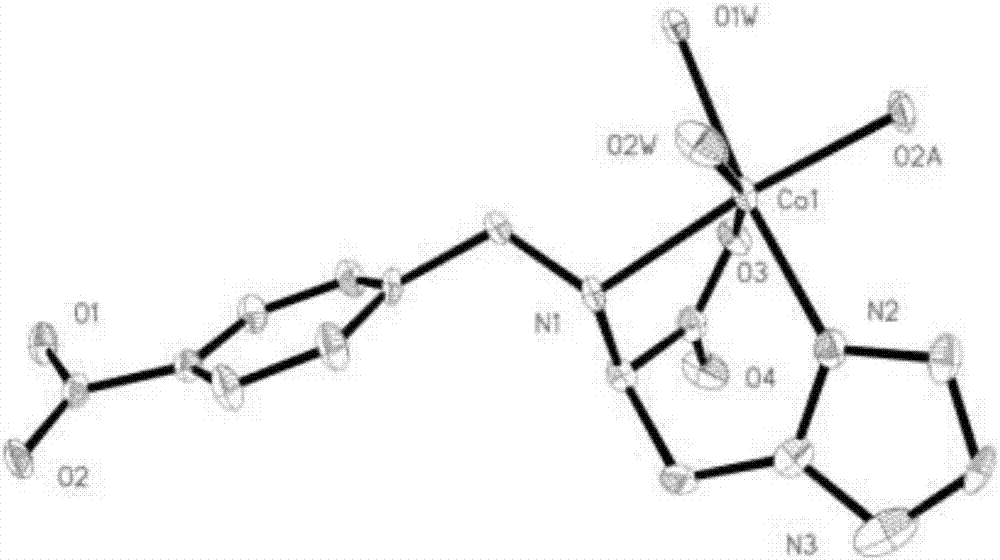
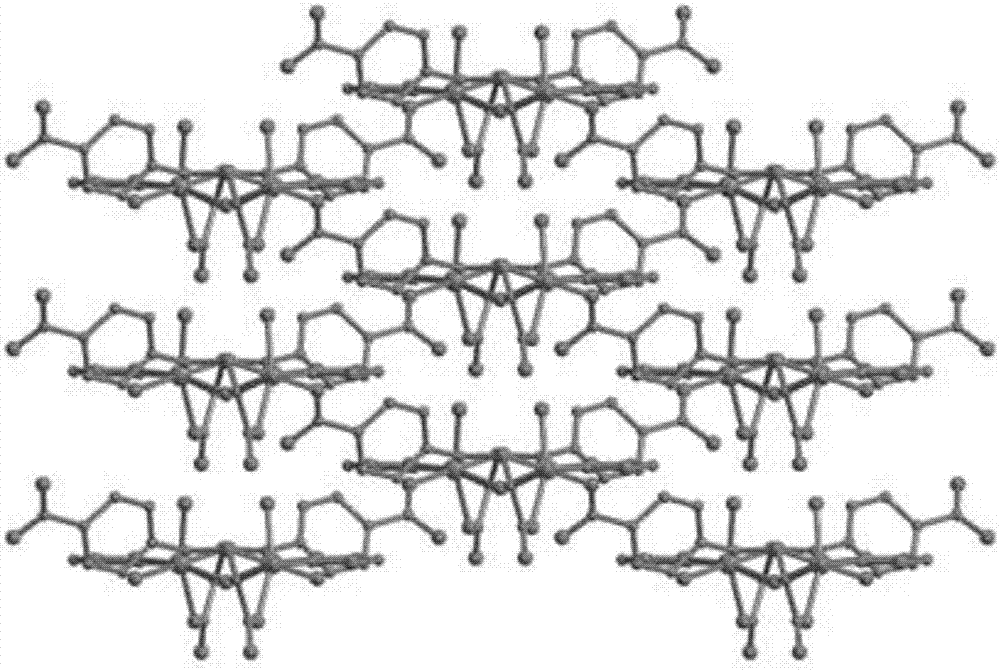
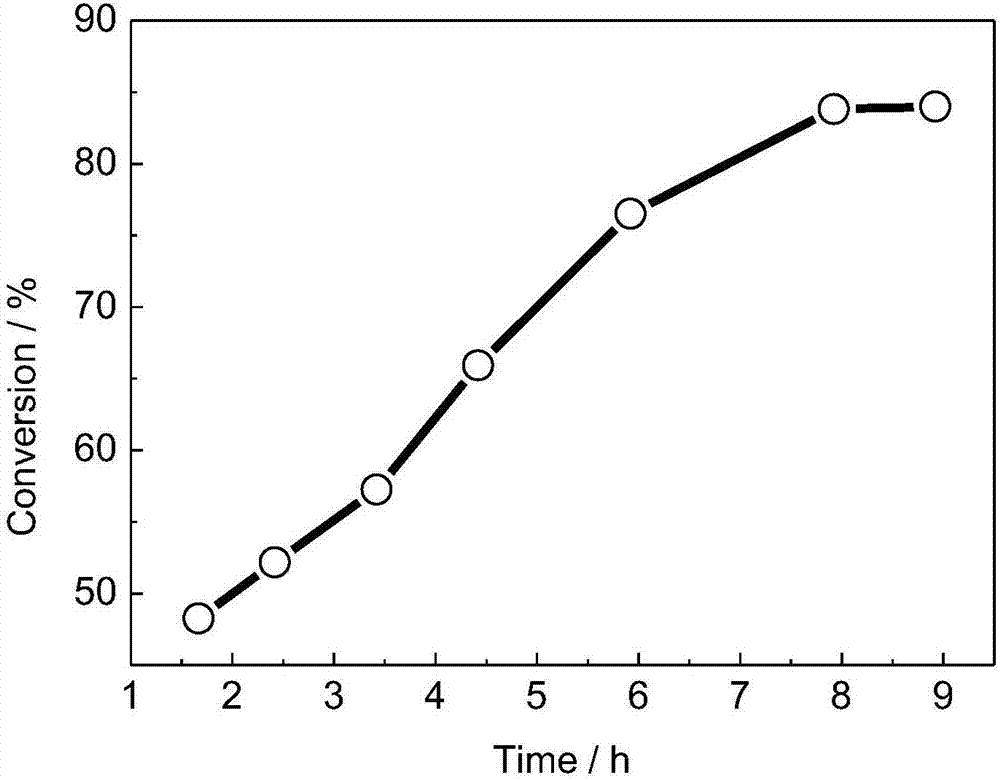
Cobalt coordination polymer constructed by histidine derived ligand and preparation method of cobalt coordination polymer
A technology of coordination polymer and histidine is applied in the field of cobalt coordination polymer constructed by histidine-derived ligands and its preparation, which can solve the problem of high synthesis cost, hindering the stretching of coordination structure, and water stability of coordination material. problems such as poor performance, to achieve the effect of high conversion rate and high stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Preparation method of histidine-derived ligand:
[0030] 1) Weigh 0.32 g of sodium hydroxide solid in a flask, measure 3 mL of distilled water, and stir until completely dissolved; weigh 1.1 g of L-histidine into a three-necked flask, stir with gentle heat until all amino acids are dissolved, and obtain a light yellow solution. Weigh 0.7g of benzoic acid-4-formaldehyde and dissolve it in 3mL of ethanol to obtain a colorless solution, and add it to a three-necked flask. The reaction is stirred at room temperature for 20 minutes to obtain a yellow solution; 2) The yellow solution obtained in step 1) is divided for 5 minutes with stirring. Add 0.3 g of solid sodium borohydride twice, and stir for 30 min in an ice bath until the yellow color of the solution is completely faded to obtain a colorless solution; 3) Transfer the colorless solution obtained in step 2) to a 50 mL beaker, and evaporate part of the solvent to A large number of white crystals appear, add about 30mL ...
Embodiment 2
[0039] (1) Preparation method of histidine-derived ligand: 1) Weigh 0.36 g of sodium hydroxide solid in a flask, measure 6 mL of distilled water, and stir until completely dissolved; weigh 1.4 g of L-histidine into a three-necked flask , Stir gently until all the amino acids are dissolved to obtain a light yellow solution, weigh out 0.9 g of benzoic acid-4-formaldehyde and dissolve in 6 mL of ethanol to obtain a colorless solution, and add it to a three-necked flask, stir and react at room temperature for 40 minutes to obtain a yellow solution; 2 ) Take the yellow solution obtained in step 1) for about 5 minutes, add 0.35 g of sodium borohydride solid in two portions under stirring, and stir for 30 minutes in an ice bath until the yellow color of the solution completely fades to obtain a colorless solution; 3) add the solution obtained in step 2) The colorless solution was transferred to a 50mL beaker, and part of the solvent was evaporated under low heat until a large number of...
Embodiment 3
[0044] (1) Preparation method of histidine-derived ligand: 1) Weigh 0.34 g of sodium hydroxide solid in a flask, measure 4 mL of distilled water, and stir until completely dissolved; weigh 1.2 g of L-histidine into a three-necked flask , Stir gently until all the amino acids are dissolved to obtain a pale yellow solution. Weigh 0.8 g of benzoic acid-4-formaldehyde and dissolve it in 5 mL of ethanol to obtain a colorless solution, and add it to a three-necked flask, stir and react for 30 minutes at room temperature to obtain a yellow solution; 2) The yellow solution obtained in step 1) is added for 6 minutes with stirring. Add 0.32g of solid sodium borohydride twice, and stir for 35 minutes in an ice bath until the yellow color of the solution is completely faded to obtain a colorless solution; 3) Transfer the colorless solution obtained in step 2) to a 50mL beaker, and evaporate part of the solvent to A large number of white crystals appeared. Add about 30mL of distilled water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com