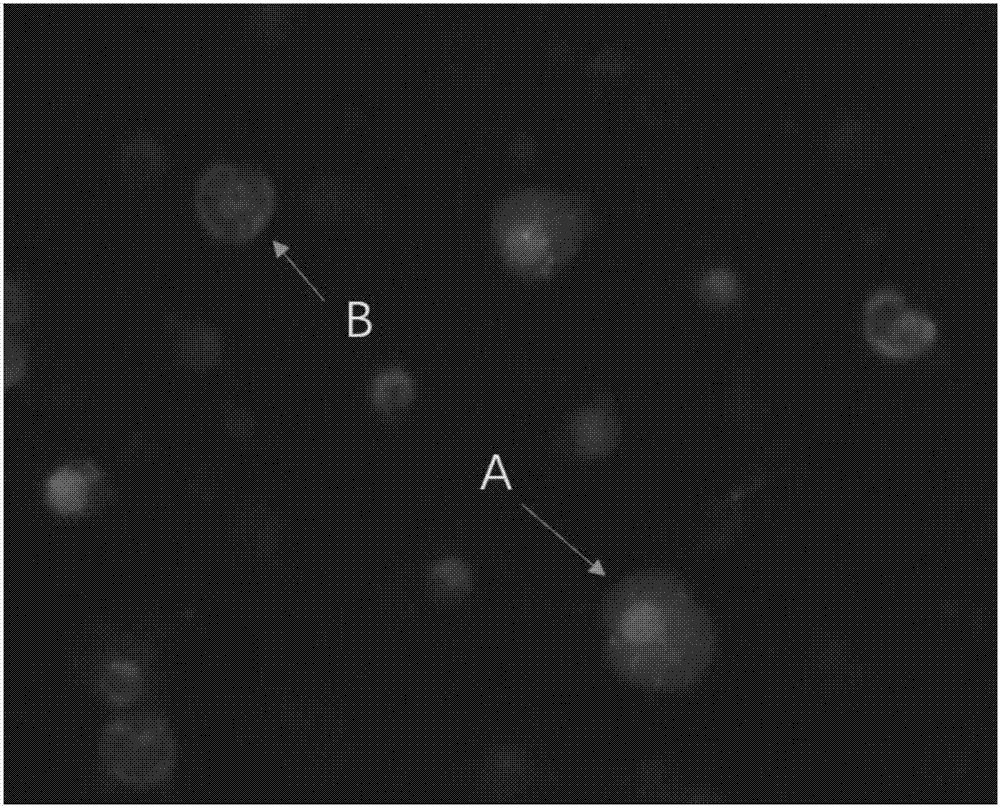
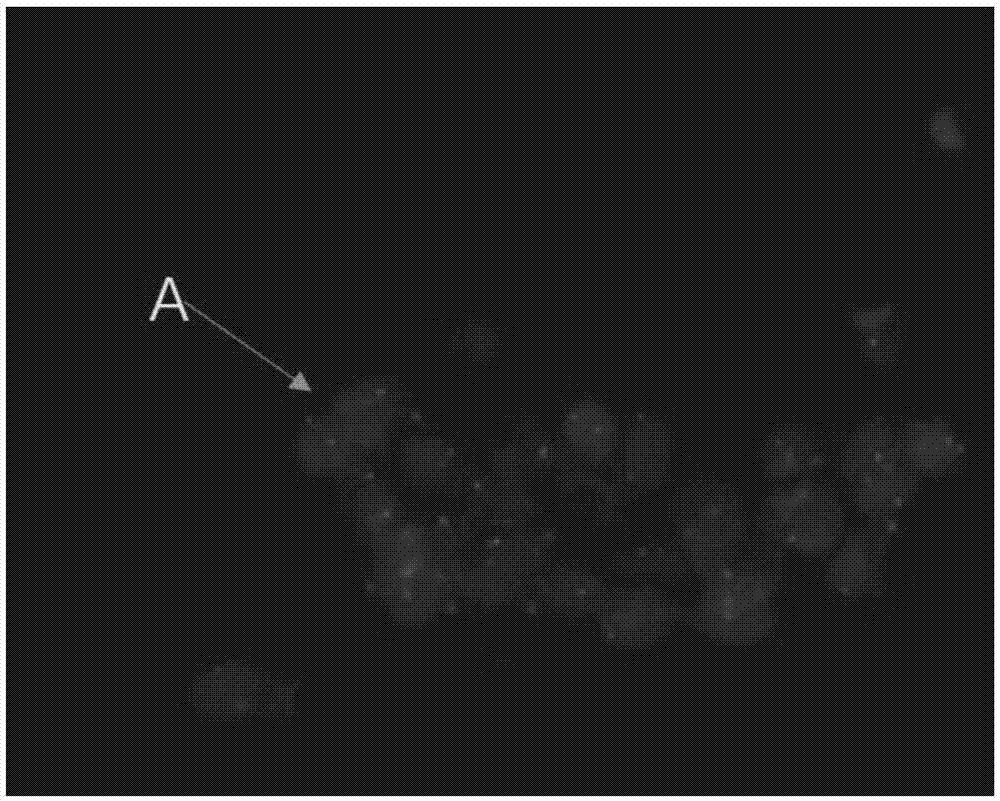
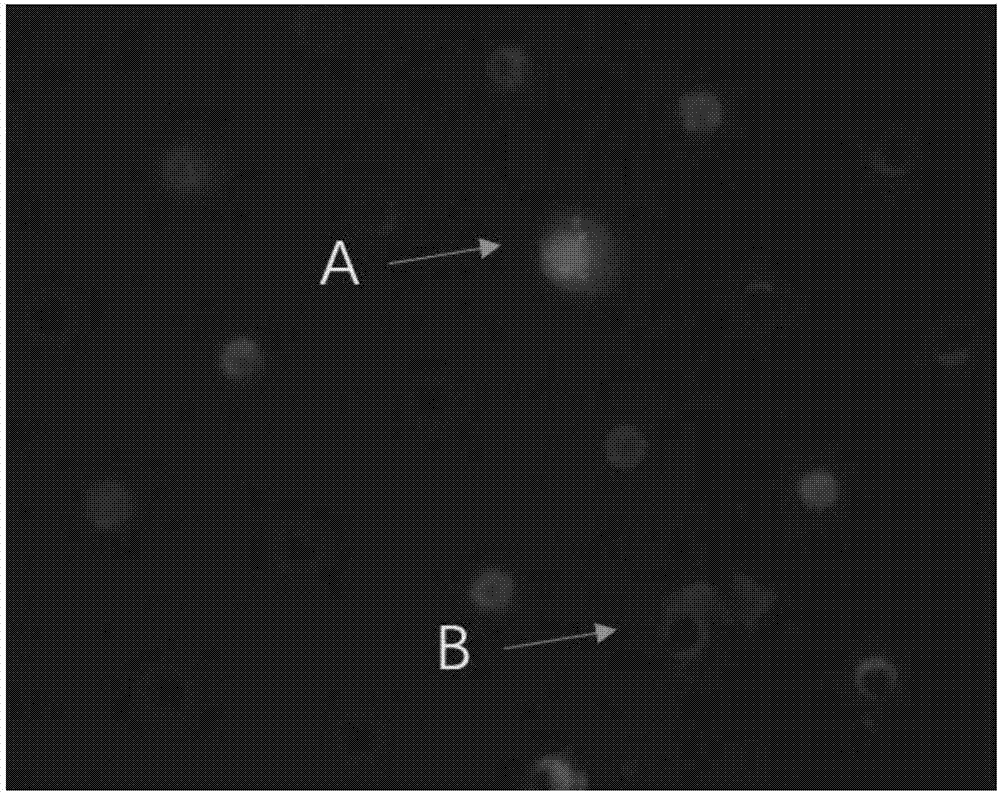
Kit for identification of circulating tumor cells through combination of CD45 immunofluorescence and CEP17 probe and use thereof
A tumor cell and immunofluorescence technology, applied in the field of biomedical clinical detection, can solve the problems of difficulty in finding target CTCs, limit the application of FISH technology, etc., and achieve the effect of reducing non-specific hybridization signals, reducing identification errors, and reducing counting errors.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A kit for identifying circulating tumor cells using CD45 immunofluorescence combined with CEP17 fluorescence in situ hybridization probe, the composition of the kit is shown in Table 1 below:
[0042] Table 1 Kit composition
[0043]
Embodiment 2
[0045] Using the kit described in Example 1 to identify circulating tumor cells in breast cancer patients, the CD45 monoclonal antibody + fluorescent in situ hybridization probe for chromosome 17 was used for detection, and the fluorescent in situ hybridization probe specific sequence for chromosome 17 (SEQ ID NO. 1) is: TGAACATTCCTTTGGATGGAGCAGGTTTGAGACACTCTTTTTGTACAATCTACAAGTGGATATTTGGACCTCCTCTGAGGATTTCGTTGGAAACGGGATAACTGCACCTAACTAAACGGAAGCATTCTCAGAAACTTCTTGGTGATGTTTGCATTCAAATCCCAGAGT
[0046] Specifically include the following steps:
[0047] (1) Harvest cells: suck the captured circulating tumor cell samples (CTCs) into a conical centrifuge tube, centrifuge at 1000 rpm for 10 min, and remove the supernatant;
[0048] (2) Hypotonicity: Add 6-8 mL of 0.075 mol / L KCL solution pre-warmed to 37°C, blow and mix with a straw, and place in a 37°C incubator for 20-30 minutes;
[0049] (3) Pre-fixation: Add 2 mL of fixative, mix by pipetting, and centrifuge at 1000 rpm for 10 min; ...
Embodiment 3
[0064] Using the kit described in the embodiment of the present invention, CD45 immunofluorescence and CEP17 fluorescence in situ hybridization were used to jointly detect circulating tumor cells in patients with gastric cancer. The specific sequence of chromosome 17 fluorescence in situ hybridization probe (SEQ ID NO.1) is:
[0065] TGAACATTCCTTTGGATGGAGCAGGTTTGAGACACTCTTTTTGTACAATCTACAAGTGGATATTTGGACCCTCTCTGAGGATTTCGTTGGAAACGGGATAACTGCACCTAACTAAACGGAAGCATTCTCAGAAACTTCTTGGTGATGTTTGCATTCAAATCCCAGAGT
[0066] Specifically include the following steps:
[0067] (1) Harvesting cells: extract 10ml of peripheral blood from the patient, and use a commercially available circulating tumor cell capture instrument or kit to capture circulating tumor cells (CTCs). The captured circulating tumor cells are sucked into a conical centrifuge tube, centrifuged, and rotated at 1000 / min, 10min, remove the supernatant;
[0068] (2) Hypotonicity: Add 6-8 mL of 0.075mol / L KCL solution pre-warmed t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com