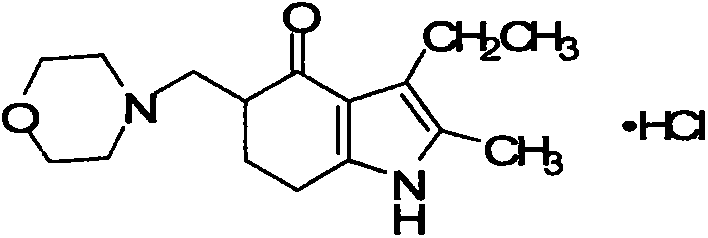
A kind of synthetic method of improved molindone
A synthesis method and technology of indanone are applied in the field of synthesis of antipsychotic drug indanone, which can solve the problems of energy consumption and time consuming in the post-treatment process of dilute acetic acid mother liquor, and achieve the effects of simplifying the subsequent complexity, reducing waste water and improving the environment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
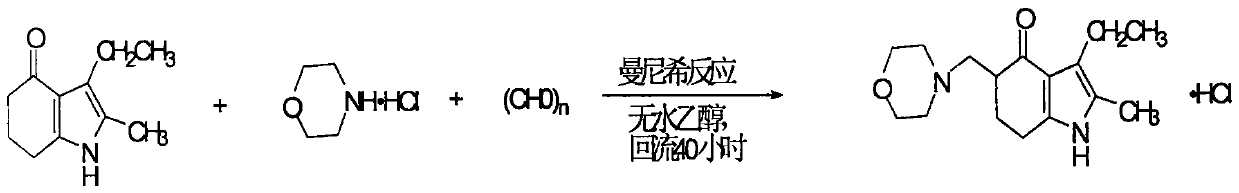
[0040] 1) Condensation reaction
[0041] Put 715ml of methanol into a 1000ml reaction bottle, start stirring, slowly add concentrated hydrochloric acid into the bottle with a dropping funnel, and adjust the pH value of the solution to 3.5-3.8. After the addition, continue to stir for 5 minutes until the retest remains unchanged. Then add 89.5g (purity 0.5mol) of raw material 2-methyl-3-ethyl-4,5,6,7-tetrahydroindolin-4-one, 81.15g (purity 0.65mol) of hydrochloric acid Morpholine and 22.75g (in terms of formaldehyde, 0.75mol) paraformaldehyde), after the addition, the water bath was heated to 50°C, and kept stirring at 50-55°C for 5h, then heated to boiling, and refluxed for 1.25hr to reach Reaction endpoint (determined by HPLC method).
[0042] 2) Water analysis
[0043] After the reaction was completed, the reaction solution was poured into 2550 ml of normal temperature water while stirring. Continue to stir for 10min, and then let it stand for 2h. The unreacted raw mate...
Embodiment 2
[0052] Embodiment 2 (pilot production example)
[0053] 1) Reaction: Put 115kg of methanol into a 200L glass-lined reactor equipped with a reflux condenser and a pH online detector at a time, and then slowly add reagent hydrochloric acid into the reactor through a dropping funnel until the pH value of the reaction solution reaches 3.65 Stop the dropwise addition and continue to stir for 10 minutes until the pH value of the retest remains unchanged. Then drop into the reactor 17.9kg (100.0mol.) 2-methyl-3-ethyl-4,5,6,7-tetrahydroindolin-4-one, 16.85kg (135.mol) hydrochloric acid morphine and 4.55 kg (151.5 mol) of paraformaldehyde. After the addition, the temperature of the water bath was raised to 50°-52°C and the reaction was stirred for 6 hours, then the temperature was raised to boiling, and the reaction was refluxed for 1.75 hours, and the end point was determined by HPLC.
[0054] 2) Water analysis and impurity removal: After the reaction is completed, drain the hot wat...
Embodiment 3
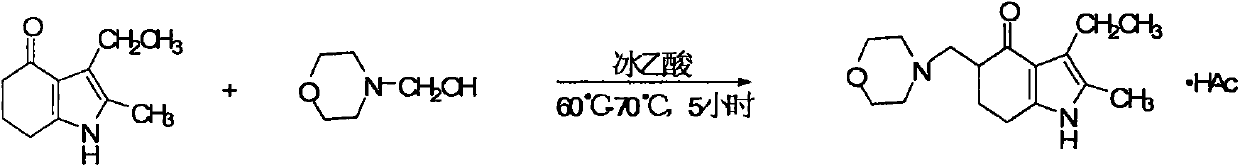
[0058] Embodiment 3 (reaction medium comparative example in embodiment 1)
[0059] Change the acidic methanol in Example 1 into equal volume, acidic anhydrous ethanol with the same pH value, and increase the heat preservation reaction temperature to 70 ° C ~ reflux temperature, and all other process conditions are exactly the same as Example 1, so Obtained unreacted raw material, alkalized crude product and ethanol primary recrystallization product yield and purity result are as follows:
[0060] 1) Recover 24.2 g of off-white powder unreacted raw material—2-methyl-3-ethyl-4,5,6,7-tetrahydroindolin-4-one, purity: 95.6%. The pure recovery rate: 26.1%.
[0061] 2) 87.9 g of white molindone crude product was obtained after alkalization of ammonia water, content: 96.1%, and condensation reaction yield: 82.7%.
[0062] 3) Take 84.5 g of the crude product, and recrystallize once with absolute ethanol according to the method of Example 1, to obtain 74.1 g of white needle-like molin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com