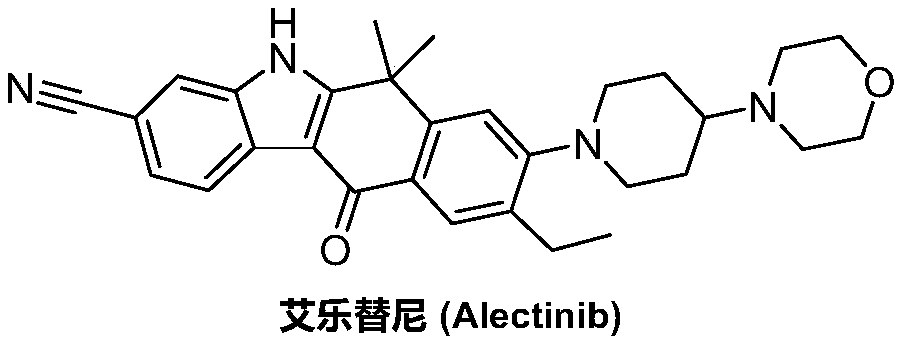
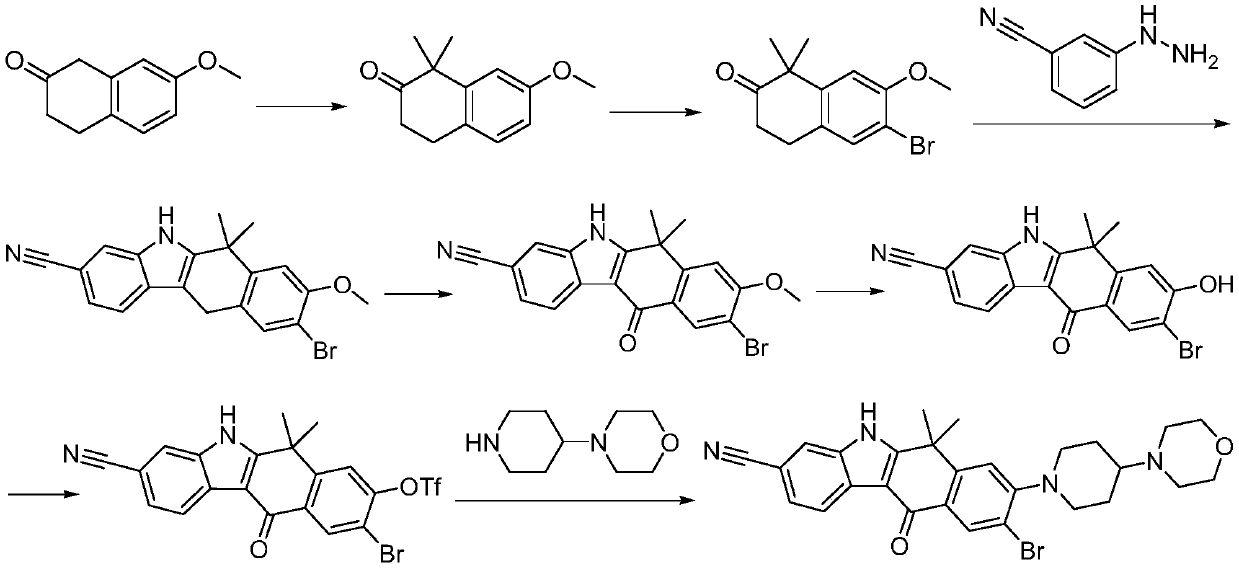
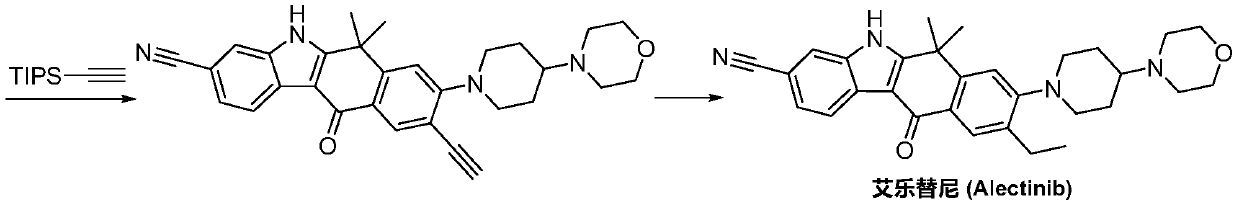
A kind of preparation method of alectinib
A technology of alectinib and piperidine, which is applied in the field of medicinal chemical synthesis, can solve the problems of short technological process, difficult to obtain, and use a large amount of solvents, and achieves the effects of reasonable technical solution, simplified operation and less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A) Preparation of 2-{4-bromo-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-2-methylpropanal:
[0042] 2-{4-Bromo-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-2-methylpropanal (6.0g, 13.7mmol) was dissolved in tetrahydrofuran (80mL) , cooled to -78°C, slowly added a toluene solution of bis(2-methoxyethoxy) sodium aluminum hydride (3.9g, 19.3mmol) dropwise, kept at -78°C and stirred for 2 hours, then evaporated to dryness under reduced pressure , added dilute hydrochloric acid to adjust to neutrality, extracted with ethyl acetate, washed with water and dried, rotary evaporated to dryness under reduced pressure, and recrystallized from isopropanol to obtain 2-{4-bromo-3-[4-(morpholine-4- yl)piperidin-1-yl]phenyl}-2-methylpropanal, pale yellow solid (4.5g), yield 83%.
[0043] B) Preparation of tert-3-chloro-4-{4-bromo-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl-2-oxopentanoic acid Butyl esters:
[0044] 2-{4-bromo-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-2-meth...
Embodiment 2
[0054] A) Preparation of 2-{4-bromo-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-2-methylpropanal:
[0055] 2-{4-bromo-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-2-methylpropanal (10.0g, 22.8mmol) dissolved in 1,4- Dioxane (150mL), cooled to -78°C, slowly added diisobutylaluminum hydride (3.9g, 27.4mmol) in n-hexane solution dropwise, kept at -78°C and stirred for 3 hours, then evaporated to dryness under reduced pressure , added dilute hydrochloric acid to adjust to neutrality, extracted with ethyl acetate, washed with water and dried, rotary evaporated to dryness under reduced pressure, and recrystallized from isopropanol to obtain 2-{4-bromo-3-[4-(morpholine-4- yl)piperidin-1-yl]phenyl}-2-methylpropanal, light yellow solid (8.7g), yield 97%.
[0056] B) Preparation of tert-3-chloro-4-{4-bromo-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl-2-oxopentanoic acid Butyl esters:
[0057]2-{4-bromo-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-2-methylpropanal (8.5g, 21.5mmo...
Embodiment 3
[0067] A) Preparation of 2-{4-bromo-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-2-methylpropanal:
[0068] 2-{4-bromo-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-2-methylpropanal (8.5g, 19.3mmol) dissolved in methyl tert-butyl Diisobutylaluminum hydride (4.3g, 30.2mmol) in n-hexane solution was slowly added dropwise, stirred and reacted at -68°C for 1 hour, then rotary evaporated to dryness under reduced pressure, added Adjust dilute hydrochloric acid to neutrality, extract with ethyl acetate, wash with water and dry, evaporate to dryness under reduced pressure, and recrystallize from isopropanol to obtain 2-{4-bromo-3-[4-(morpholin-4-yl) Piperidin-1-yl]phenyl}-2-methylpropanal, pale yellow solid (7.0 g), yield 92%.
[0069] B) Preparation of tert-3-chloro-4-{4-bromo-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl-2-oxopentanoic acid Butyl esters:
[0070] 2-{4-bromo-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-2-methylpropanal (7.0g, 17.7mmol) and 2,2-di Tert-butyl ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com