CAR-NK cell as well as preparation method and application thereof
A kind of NK cell and cell technology, applied in the biological field, can solve the problems that the application precedent of CAR-NK cell tumor immunotherapy has not been established, and achieve the effect of wide application range and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
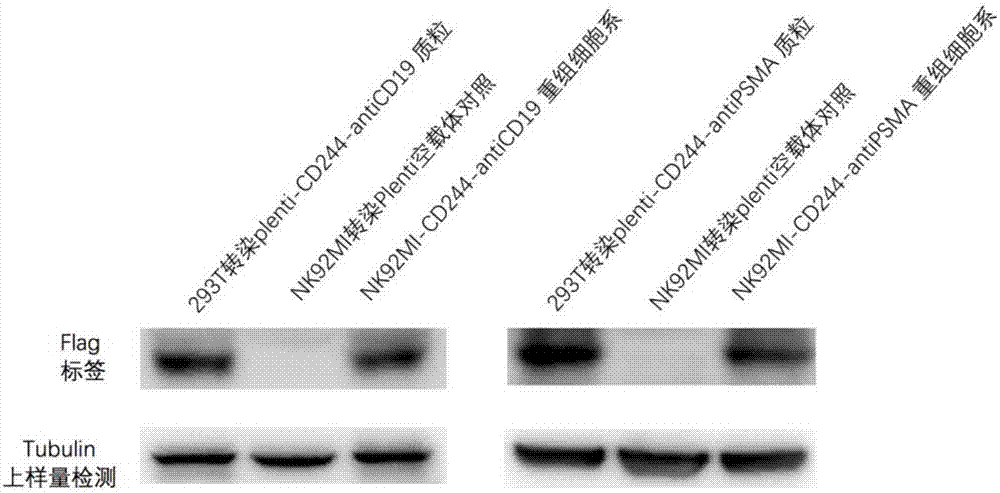
Image
Examples
Embodiment 1
[0054] Example 1. Preparation of recombinant vector CAR-CD244-antiCD19 and recombinant vector CAR-CD244-antiPSMA
[0055] 1. The specific antibody-encoding gene is amplified by PCR using primers with BfuAI restriction endonuclease recognition sites at the end;
[0056] The specific antibody coding gene sequences used in this example are the partial sequence of CD19 antibody (antiCD19) (sequence listing sequence 2) and the partial sequence of PSMA antibody (antiPSMA) (sequence listing sequence 3);
[0057] 2. Purify the PCR products of step 1 respectively;
[0058] 3. Carry out single enzyme digestion (BfuAI) to the PCR product purified in step 2, and simultaneously carry out single enzyme digestion (BfuAI) to the cloning vector containing the transformed CD244 backbone;
[0059] 4. Purify the digested product;
[0060] 5. After the purified product is prepared according to the molar ratio of the antibody fragment and the cloning carrier containing the modified CD244 backbone...
Embodiment 2
[0063] Embodiment 2, preparation of recombinant lentiviral vector
[0064] 1. Using the recombinant vector CAR-CD244-antiCD19 prepared in Example 1 and the recombinant vector CAR-CD244-antiPSMA as templates respectively, PCR amplification was performed using primers with BamHI and XbaI restriction endonuclease site sequences;
[0065] 2. Purify, digest (BamHI and XbaI) and purify the PCR products respectively;
[0066] 3. Use T4 ligase to connect the products of step 2 into the Plenti lentiviral vector (named pLentiCMV / TO eGFP Puro (w159-1), purchased from addgene, website: http: / / www.addgene.org / 17481 / ) between BamHI and XbaI sites;
[0067] 4. Transformation, plating, picking single clones to extract plasmids for sequencing, and named the recombinant lentiviral vectors with correct sequencing as recombinant lentiviral vectors Plenti-CAR-CD244-antiCD19 and Plenti-CAR-CD244-antiPSMA respectively.
[0068] The schematic diagram of the recombinant lentiviral vector Plenti-CAR...
Embodiment 3
[0069] Example 3, preparation and concentration of lentivirus
[0070] 1. Preparation of lentivirus
[0071] Use the recombinant lentiviral vector Plenti-CAR-CD244-antiCD19 prepared in Example 2 and psPAX2 (purchased from addgene, website at https: / / www.addgene.org / 12260 / ), pMD2.G (purchased from addgene, website 293T cells (about 3X10 6 293T cells were plated in a 10cm dish), and replaced with fresh medium (DMEM medium, supplemented with 10% fetal bovine serum) after 8 hours, and then the supernatant was collected every 24 hours and added with fresh medium (DMEM medium, supplemented with 10% Fetal bovine serum), collected 3 times in total to obtain lentiviral supernatant A.
[0072] Using the prepared recombinant lentiviral vector Plenti-CAR-CD244-antiPSMA and psPAX2, pMD2.G vectors prepared in Example 2, the mass ratio was 5ug:3.2ug:1.8ug to transfect 293T cells (about 3×10 6 293T cells were plated in a 10cm dish), and replaced with fresh medium (DMEM medium, supplemented...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com