Benzodifuranone compound with antitumor activity and preparation method and application thereof
A technology for benzodifuranone and anti-tumor activity, which is applied in the field of compounds and can solve the problems of low bioavailability, complex structure and little research.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Extraction and separation of benzodifuranone compounds
[0032] Preparation of strains: use seawater PDA medium, sterilize at high temperature, make a plate, place it at room temperature, inoculate the activated Aspergillus terreus CC-S06-18 as a strain; the seawater PDA medium The ingredients are 200g of potatoes, 20g of glucose, 15-20g of agar, and 1L of seawater;
[0033] The deposit information of Aspergillus terreus CC-S06-18 is as follows:
[0034] Preserved strains: Aspergillus terreus CC-S06-18,
[0035] Deposit unit: China Center for Type Culture Collection,
[0036] Preservation address: Wuhan University, Wuhan, China,
[0037] Deposit number: CCTCC No.M2019793,
[0038] Deposit date: October 10, 2019.
[0039] Preparation of fermented seed liquid: put seawater PDA liquid culture medium (200g of potatoes, 20g of glucose, 1L of seawater) into several Erlenmeyer flasks respectively, after high-temperature sterilization, inoculate the above-mentio...
Embodiment 2
[0043] Example 2: Structural Analysis of Benzodifuranone Compounds
[0044] The structures of benzodifuranones were determined based on their mass spectrometry and nuclear magnetic resonance data analysis.
[0045] Irregular dark red powder; NMR data are shown in the table (measured at 600MHz); molecular formula C 20 h 18 o 4 , high resolution mass spectrum HRESIMS(positive)m / z 345.1106[M+Na] + (theoretical molecular formula is C 20 h 18 o 4 Na, the theoretical molecular weight is 345.1103).
[0046] The structure is:
[0047]
[0048] NMR data are shown in Table 1.
[0049] Table 1 NMR data sheet
[0050] position δ C ,Type
Embodiment 3
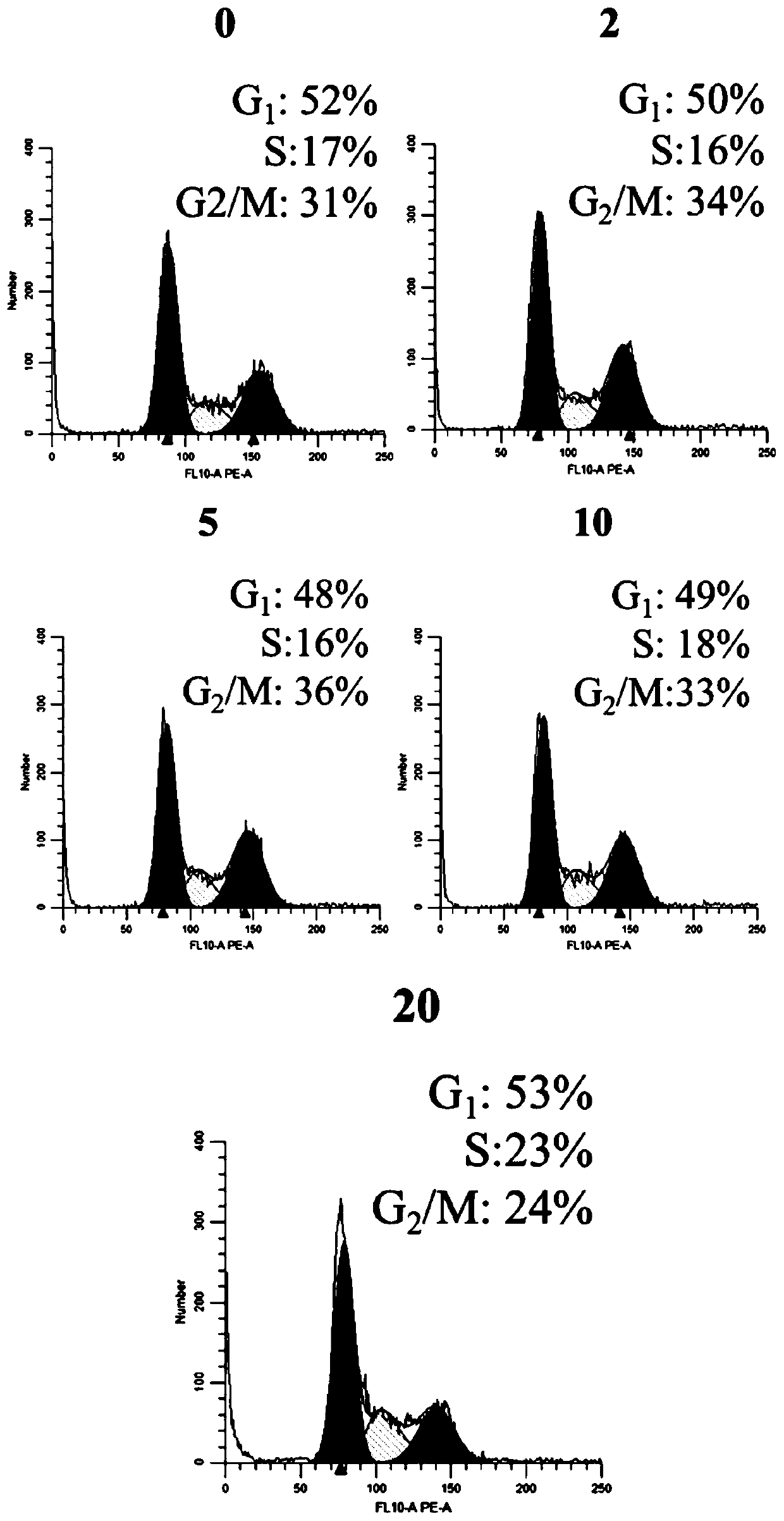
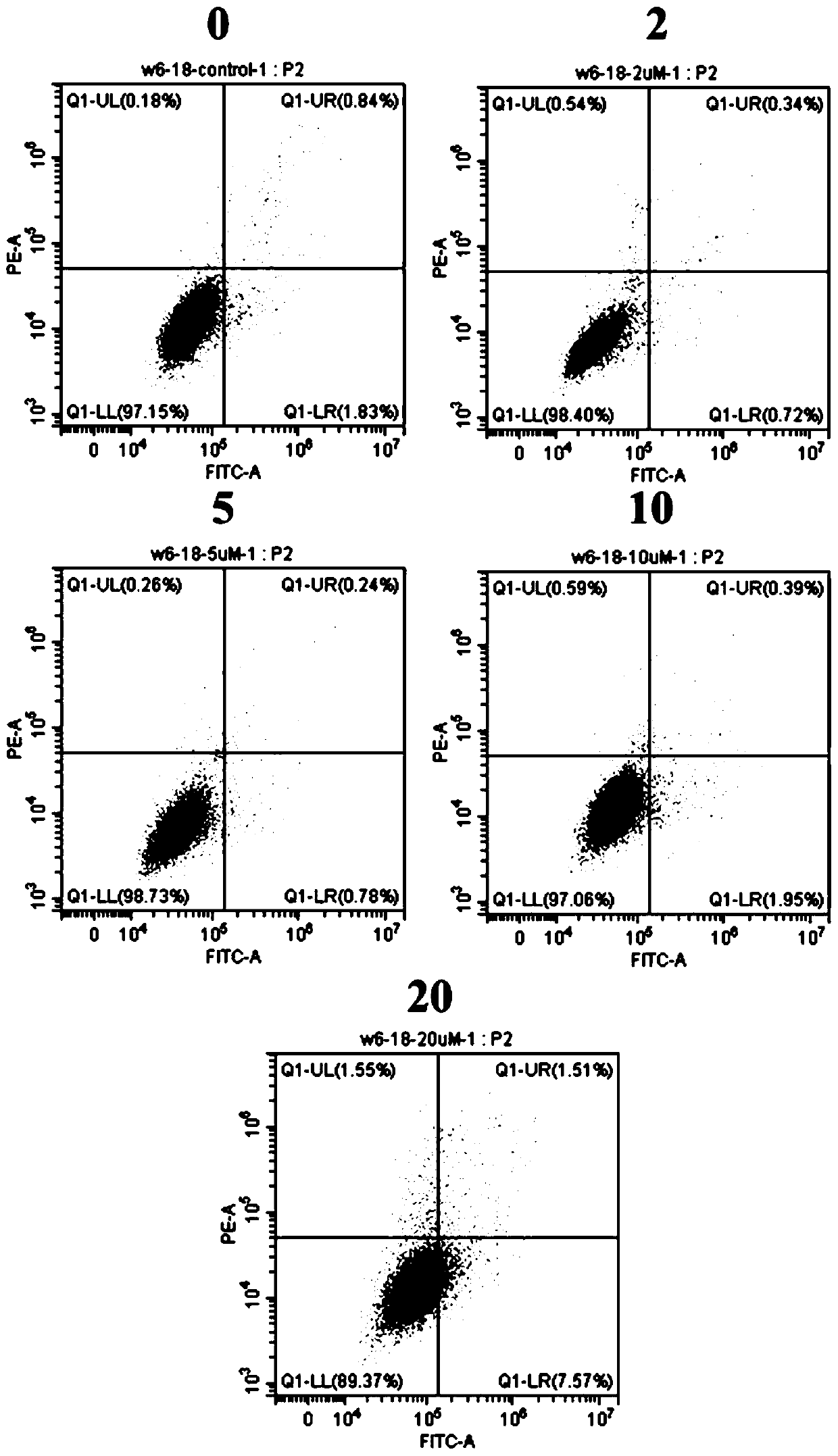
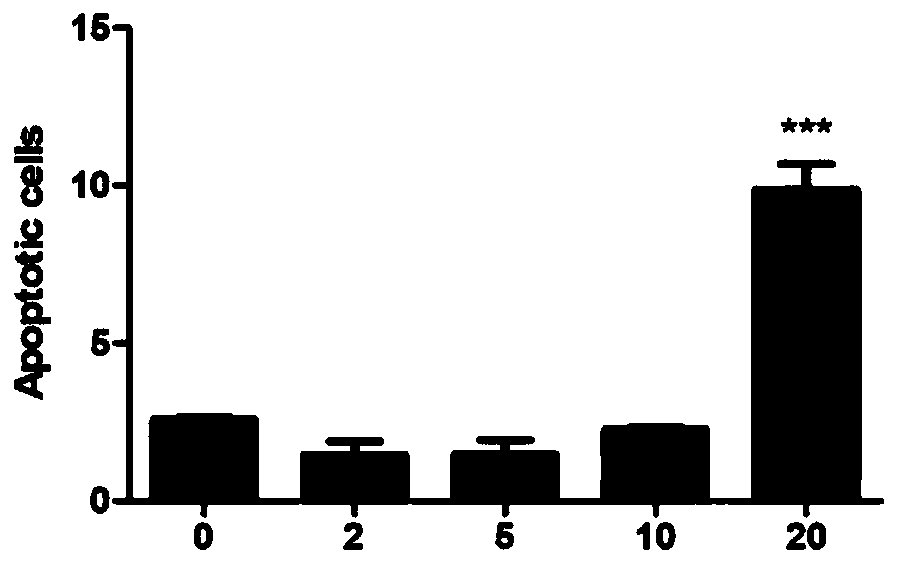
[0051] Embodiment 3: Function test of benzodifuranone compound AtrA
[0052] 1. AtrA cytotoxicity test
[0053] CCK8 assay was used to determine the effect of compound AtrA on the viability of gastric cancer cell lines HGC27, MGC803, BGC823, AGS and normal gastric epithelial cell line GES-1.
[0054] Culture of human gastric cancer cell lines:
[0055] Human gastric cancer cell lines MGC803, BGC823, AGS, HGC27 and human normal gastric epithelial cell line GES-1 were obtained from the Institute of Cells, Chinese Academy of Sciences (Shanghai, China). These cells are commercially available and can also be purchased from the ATCC. All cell lines were cultured in RPMI1640 containing 10% fetal bovine serum and 1% penicillin / streptomycin at 37 °C, 5% CO 2 cultured in an incubator.
[0056] Cell viability assay: Gastric cancer cell lines MGC803, BGC823, AGS and HGC27 and normal gastric epithelial cell line GES-1 were cultured overnight in 96-well plates, and then treated with dif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com