Preparation method of recombinant vectors CAR-CD244-antiPSMA
一种car-cd244-antipsma、CD244的技术,应用在重组载体CAR-CD244-antiPSMA的制备领域,能够解决尚未建立CAR-NK细胞肿瘤免疫治疗应用先例等问题,达到使用范围广、制备成本低的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
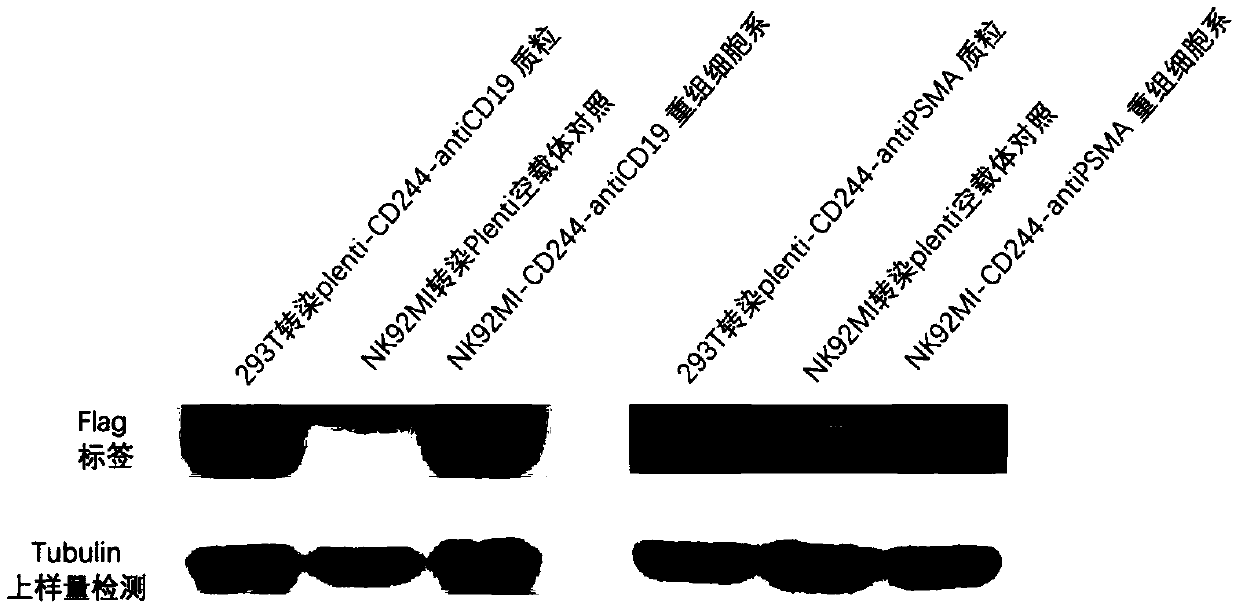
[0055] Example 1. Preparation of recombinant vector CAR-CD244-antiCD19 and recombinant vector CAR-CD244-antiPSMA
[0056] 1. The specific antibody-encoding gene is amplified by PCR using primers with BfuAI restriction endonuclease recognition sites at the end;
[0057] The specific antibody coding gene sequences used in this example are the partial sequence of CD19 antibody (antiCD19) (sequence listing sequence 2) and the partial sequence of PSMA antibody (antiPSMA) (sequence listing sequence 3);
[0058] 2. Purify the PCR products of step 1 respectively;
[0059] 3. Carry out single enzyme digestion (BfuAI) to the PCR product purified in step 2, and simultaneously carry out single enzyme digestion (BfuAI) to the cloning vector containing the transformed CD244 backbone;
[0060] 4. Purify the digested product;
[0061] 5. After the purified product is prepared according to the molar ratio of the antibody fragment and the cloning carrier containing the modified CD244 backbone...
Embodiment 2
[0064] Embodiment 2, preparation of recombinant lentiviral vector
[0065] 1. Using the recombinant vector CAR-CD244-antiCD19 prepared in Example 1 and the recombinant vector CAR-CD244-antiPSMA as templates respectively, PCR amplification was performed using primers with BamHI and XbaI restriction endonuclease site sequences;
[0066] 2. Purify, digest (BamHI and Xba I) and purify the PCR products respectively;
[0067] 3. Use T4 ligase to connect the products of step 2 into the Plenti lentiviral vector (named pLentiCMV / TO eGFP Puro (w159-1), purchased from addgene, website: http: / / www.addgene.org / 17481 / ) between BamHI and XbaI sites;
[0068] 4. Transformation, plating, picking single clones to extract plasmids for sequencing, and named the recombinant lentiviral vectors with correct sequencing as recombinant lentiviral vectors Plenti-CAR-CD244-antiCD19 and Plenti-CAR-CD244-antiPSMA respectively.
[0069] The schematic diagram of the recombinant lentiviral vector Plenti-CA...
Embodiment 3
[0070] Example 3, preparation and concentration of lentivirus
[0071] 1. Preparation of lentivirus
[0072] Use the recombinant lentiviral vector Plenti-CAR-CD244-antiCD19 prepared in Example 2 and psPAX2 (purchased from addgene, website at https: / / www.addgene.org / 12260 / ), pMD2.G (purchased from addgene, website 293T cells (about 3×10 6 293T cells were plated on a 10cm dish), and replaced with fresh medium (DMEM medium, added with 10% fetal calf serum) after 8 hours, and the supernatant was collected every 24 hours thereafter and added with fresh medium (DMEM medium, added with 10% Fetal bovine serum), collected 3 times in total to obtain lentiviral supernatant A.
[0073] Using the recombinant lentiviral vector Plenti-CAR-CD244-antiPSMA prepared in Example 2 and the psPAX2, pMD2.G vectors in a mass ratio of 5ug:3.2ug:1.8ug to transfect 293T cells (about 3×10 6 293T cells were plated on a 10cm dish), and replaced with fresh medium (DMEM medium, supplemented with 10% fetal ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com