Process for preparing potassium sulfate by sea water
A technology of potassium sulfate and seawater, applied in chemical instruments and methods, alkali metal sulfite/sulfite, alkali metal compounds, etc., can solve the problem that the potassium reserves of salt lakes cannot meet domestic demand, the production of potassium sulfate is restricted by raw material production, Problems such as high cost of potassium raw material sources, to overcome insufficient potassium reserves, low cost, and good quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
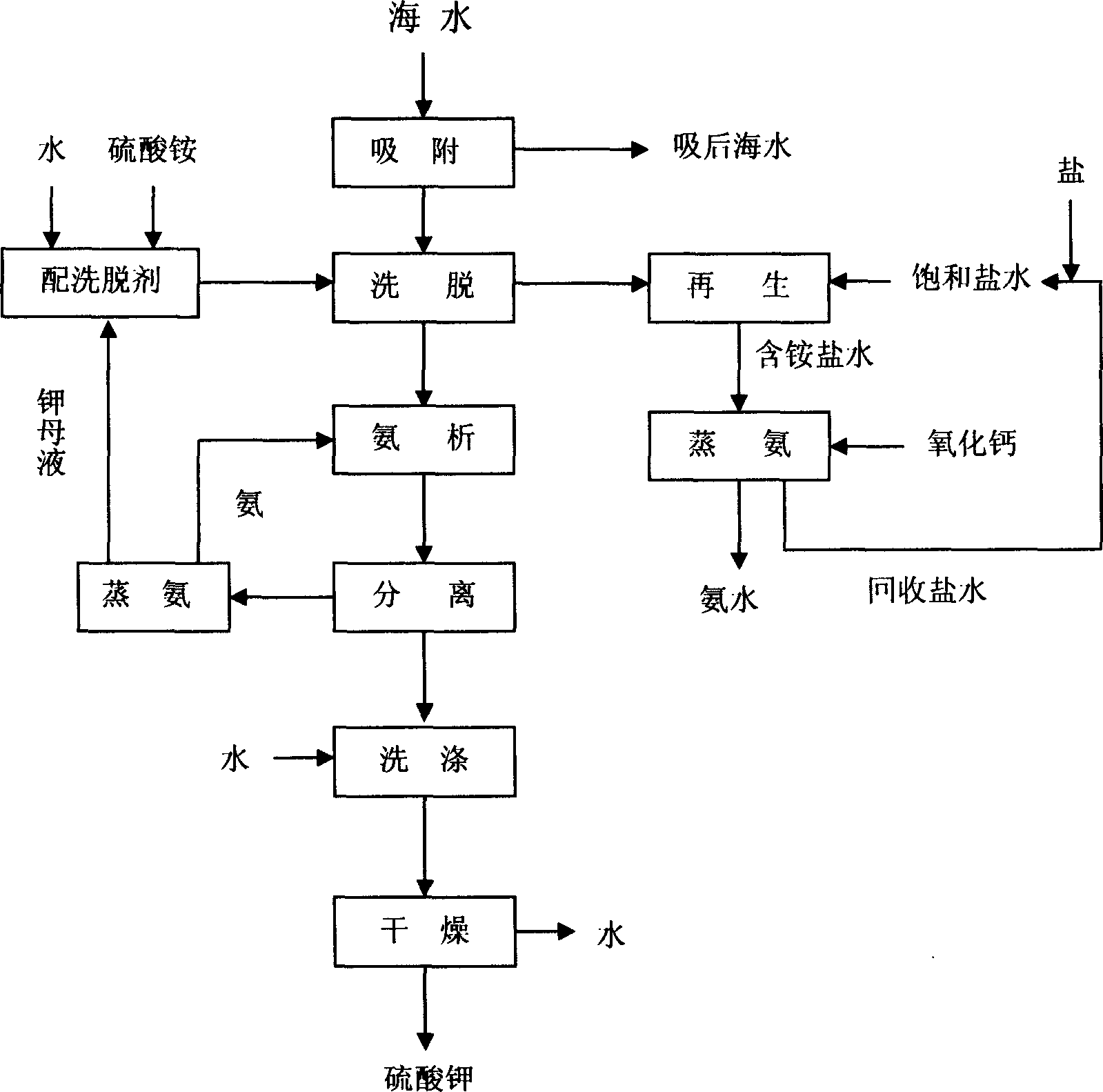
[0016] The first step, preparation of potassium-rich solution
[0017] Firstly, 4000 grams of sodium-type natural clinoptilolite is filled in a φ30×6000 mm jacketed ion exchange column to form an ion exchange device. Then feed 20 liters of raw material concentrated seawater of 25 ° of B 'e of 24166 grams with the flow velocity of 5 meters / hour to ion-exchange column, carry out adsorption, and adsorption temperature is 30 ℃. The concentration of potassium in the raw material concentrated seawater is 4.30 g / L. After adsorption, the seawater in the ion exchange column is completely ejected with fresh water, and the concentration of potassium in the discharged seawater is 1.15 g / L. Potassium ions in seawater are exchanged into zeolites. According to the data in Table 1, the potassium mother liquor and solid ammonium sulfate after water and the ammonia distillation of the last cycle are formulated as eluent, and the ion exchange column is passed into the above-mentioned ion exchan...
Embodiment 2
[0023] The first step, preparation of potassium-rich solution
[0024]Firstly, 4000 grams of sodium-type natural clinoptilolite is filled in a φ30×6000 mm jacketed ion exchange column to form an ion exchange device. Then feed 600 liters of raw material seawater of 609 kilograms of 2°B'e with a flow velocity of 50 m / h to the ion exchange column for adsorption, and the adsorption temperature is 0°C. The potassium concentration in the raw seawater is 0.32 g / L. After adsorption, the seawater in the ion exchange column is completely ejected with fresh water, and the potassium concentration in the discharged seawater is 0.16 g / L. Potassium ions in seawater are exchanged into zeolites. According to the data in Table 3, the potassium mother liquor and solid ammonium sulfate after water and the ammonia distillation of the last cycle are formulated as eluent, and the ion exchange column is passed into the above-mentioned ion exchange column with a flow velocity of 8 meters / hour. The e...
Embodiment 3
[0030] The first step, preparation of potassium-rich solution
[0031] Firstly, 4000 grams of sodium-type natural clinoptilolite is filled in a φ30×6000 mm jacketed ion exchange column to form an ion exchange device. Then feed 100 liters of raw material concentrated seawater of 109 kilograms of 12 ° B 'e with the flow velocity of 15 m / h to the ion exchange column for adsorption, and the adsorption temperature is 15 ° C. The potassium concentration in the raw seawater is 1.52 g / L. After adsorption, the seawater in the ion exchange column is completely ejected with fresh water, and the potassium concentration in the discharged seawater is 0.75 g / L. Potassium ions in seawater are exchanged into zeolites. According to the data in Table 5, the eluent is formulated with water and the mother liquor and solid ammonium sulfate after the ammonia distillation of the previous cycle, and the eluent with a temperature of 70° C. is passed into the above-mentioned ion exchange column with a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com