Globin gene therapy for treating hemoglobinopathies
A globulin and gene technology, applied in hemoglobin/myoglobin, gene therapy, blood diseases, etc., can solve problems such as being unfavorable for producing high titer vectors and effectively transducing HSC in patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0261] Example 1: Discovery of new insulators
[0262] The problems created by insertional mutagenesis of viral vectors are well known (Nienhuis (2013), Baum et al. (2006), Nienhuis et al. (2006)), as the risk of genotoxicity can be reduced by using chromatin insulators (Arumugam et al. (2007), Emery (2011), Evans-Galea et al. (2007), Rivella et al. (2000), Emery et al. (2000), Emery et al. (2002), Yannaki et al. (2002), Hino et al. ( 2004), Ramezani et al. (2003), Ramezani et al. (2008)). Methods have been developed that allow the efficient identification of enhancer-blocking insulators in the human genome. These new insulators are short, averaging 150 bp, and they do not adversely affect the titer of viral vectors, and they are several times more potent than the insulator cHS4. Using a genomic approach to discover the most powerful enhancer blockers and barrier insulators of the human genome. For gene therapy in hemoglobinopathies, strong enhancers are required to achie...
Embodiment 2
[0267] Example 2: Characterization of globulin carriers comprising at least one insulator
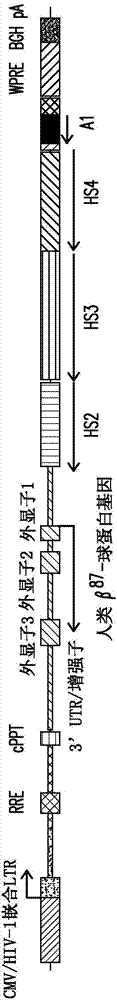
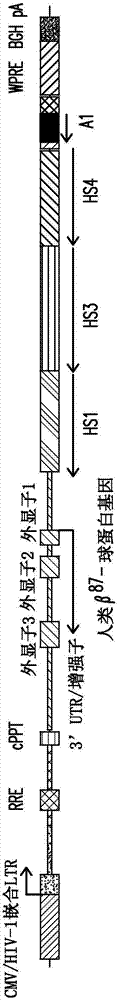
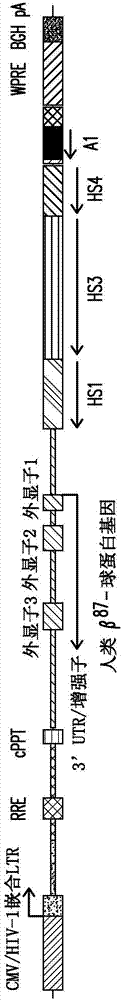
[0268] Production of the expression cassette disclosed in the present invention (referred to as "expression cassette 1", such as figure 1 shown), which contains insulator A1, and human β encoding a threonine-to-glutamine mutation at codon 87 A -globin gene (β A-T87Q ), operably linked to the β-globin LCR region, the β-globin LCR region comprises the HS2 region having the nucleotide sequence shown in SEQ ID NO:9, the nucleotide sequence having the nucleotide sequence shown in SEQ ID NO:5 The HS3 region and the HS4 region having the nucleotide sequence shown in SEQ ID NO:7. Using the variant beta chain (beta A ) rationale is to facilitate the detection of vector-encoded β-globin genes, distinguishing them from endogenous or imported β-chains. A glutamine (GLN) residue at position 87 in the γ-globulin chain enhances the antisickling activity of the γ chain relative to the β chain whi...
Embodiment 3
[0270] Example 3: Evaluation of enhancer activity in non-erythroid K562 cells
[0271] Enhancer activity of HS2 was evaluated in nonerythroid K562 cells. Such as Figure 7 GFP expression in K562 cells transduced with the vector is driven by a minimal promoter linked to no enhancer ("blank", HS2, HS3-HS4, HS2-HS3-HS4, or the runx1 enhancer used as a positive control), as shown ("RUNX1"). Background expression was on the order of 0.01% ("Blank"), but increased more than 10-fold with HS2-HS3-HS4 ("Lcr9", 0.17%). This enhancement was mainly due to HS2 (0.15%), Instead of HS3-HS4 (0.05%). All cell lines were transduced equally (average vector copy number 2.5). Results support that HS2 but not HS3-HS4 may cause oncogenic risk in non-erythroid hematopoietic stem and progenitor cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com