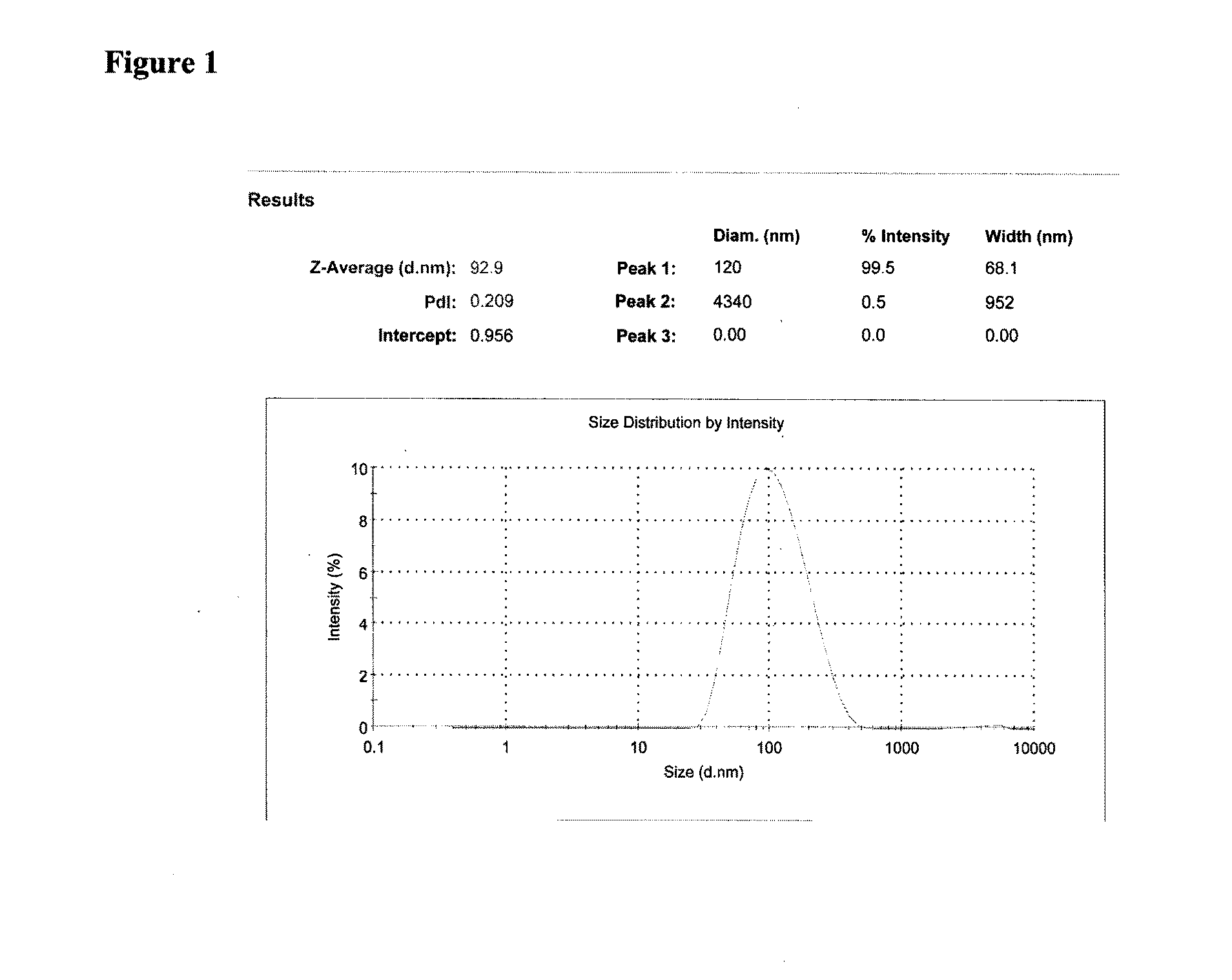
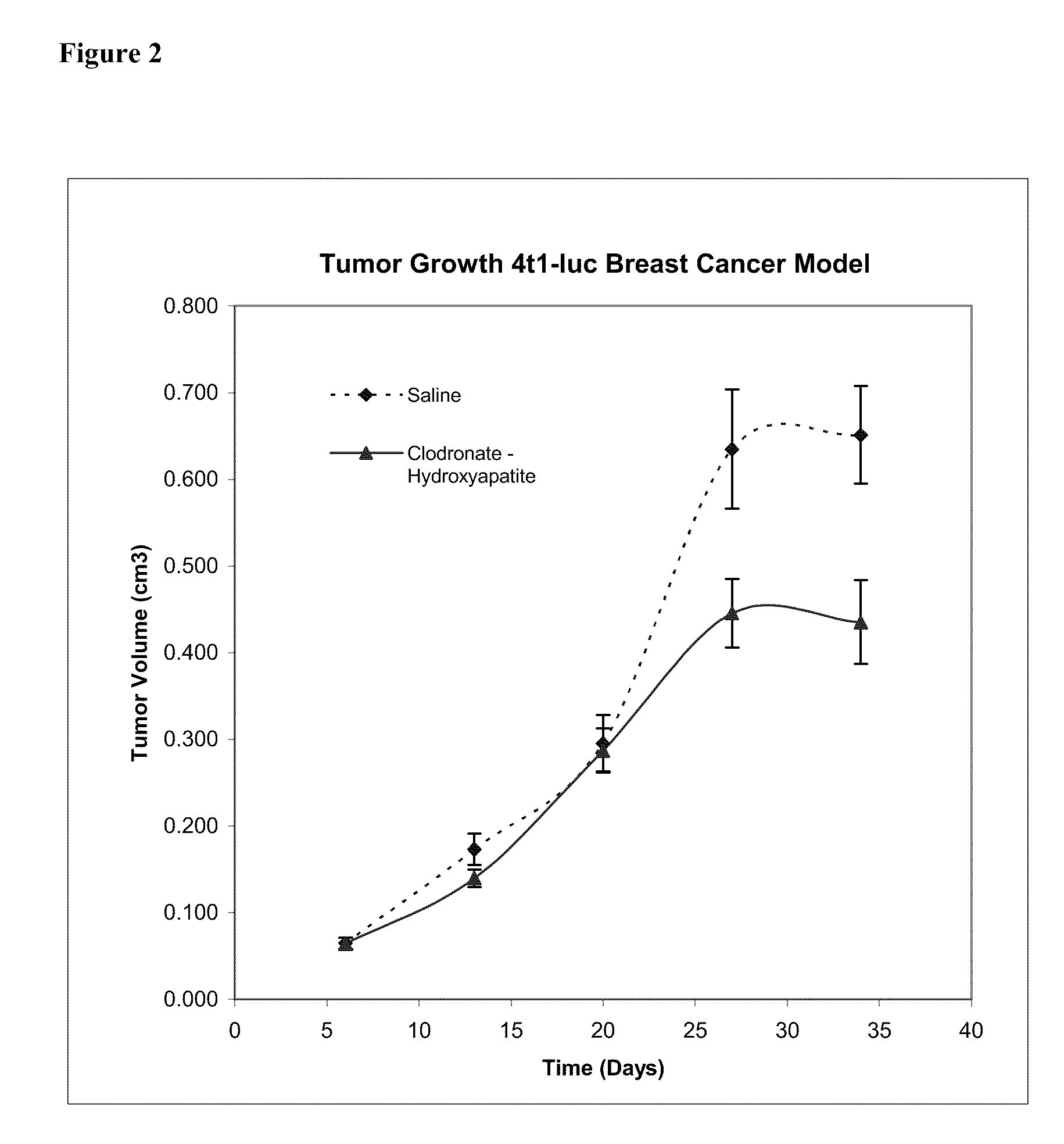
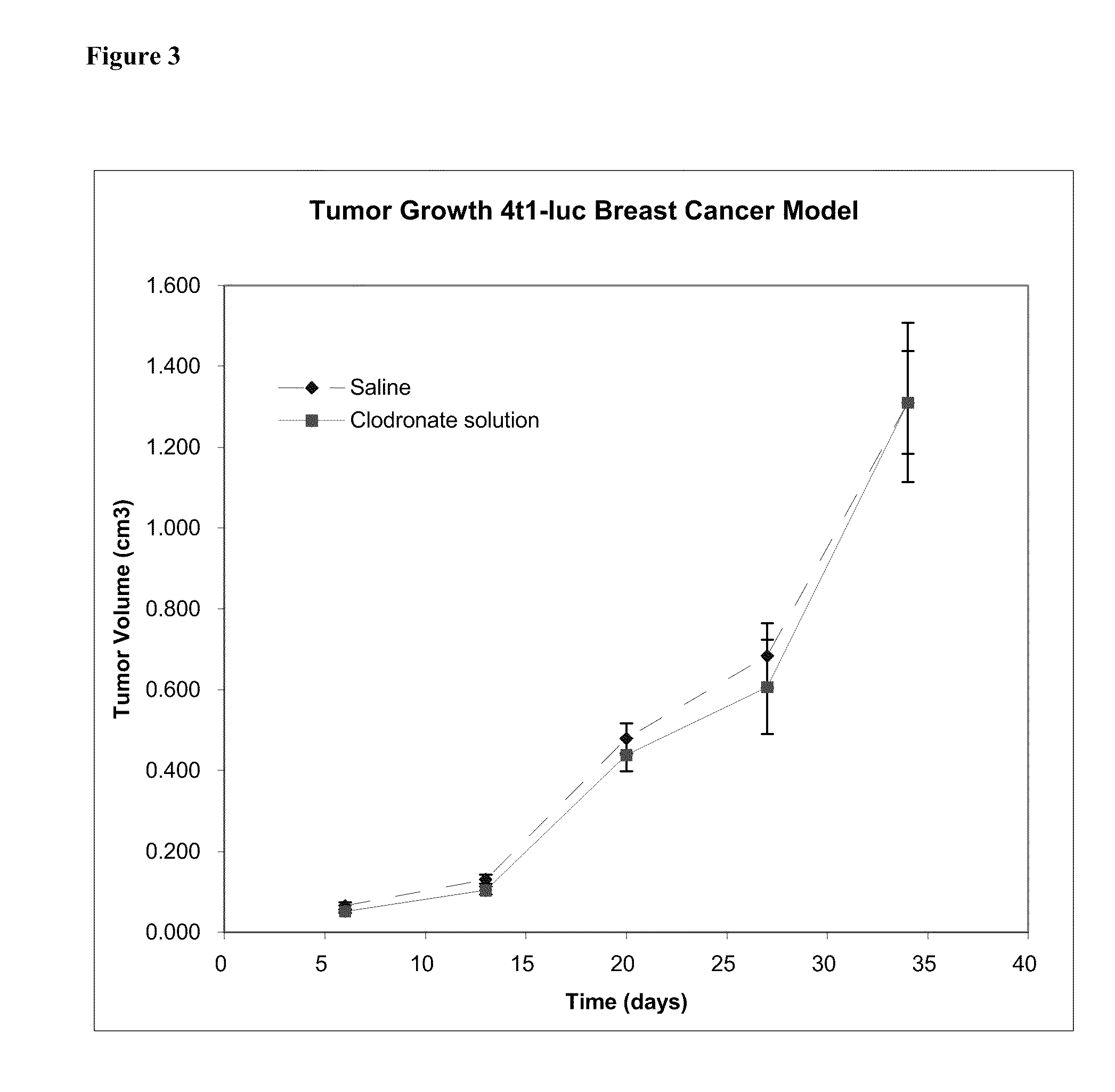
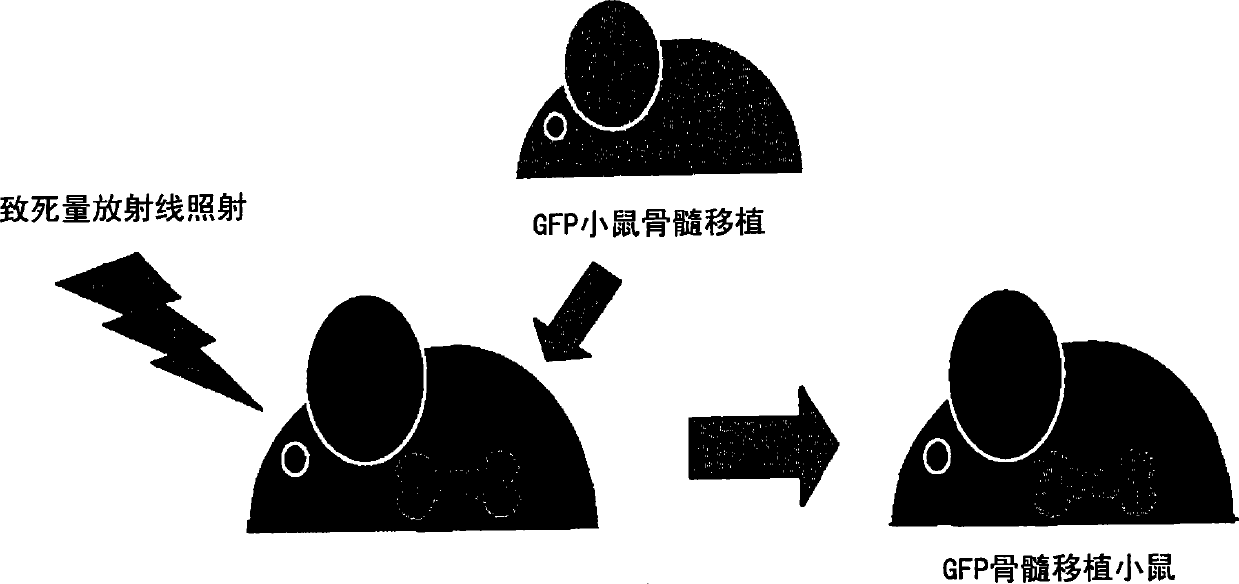
Patents
Literature
30 results about "Bone Marrow-Derived Cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A cell that is isolated from bone marrow.
Microparticle compositions to modify cancer promoting cells
This invention provides pharmaceutical compositions and methods related to the prevention and treatment of primary tumors and metastatic, malignant or spreading cancers by selectively targeting cancer associated myeloid derived cells by the targeted delivery of a bisphosphonate formulated with a non-liposomal particle carrier. In some aspects, the bisphosphonate particles have one or more properties suitable for phagocytosis by cancer associated myeloid derived cells and release of the bisphosphonate within the macrophages. Advantageously, administering the particles to a subject reduces the level and / or activity of cancer associated myeloid derived cells in the subject.
Owner:JOVESIS
Pharmaceutical for promoting functional regeneration of damaged tissue
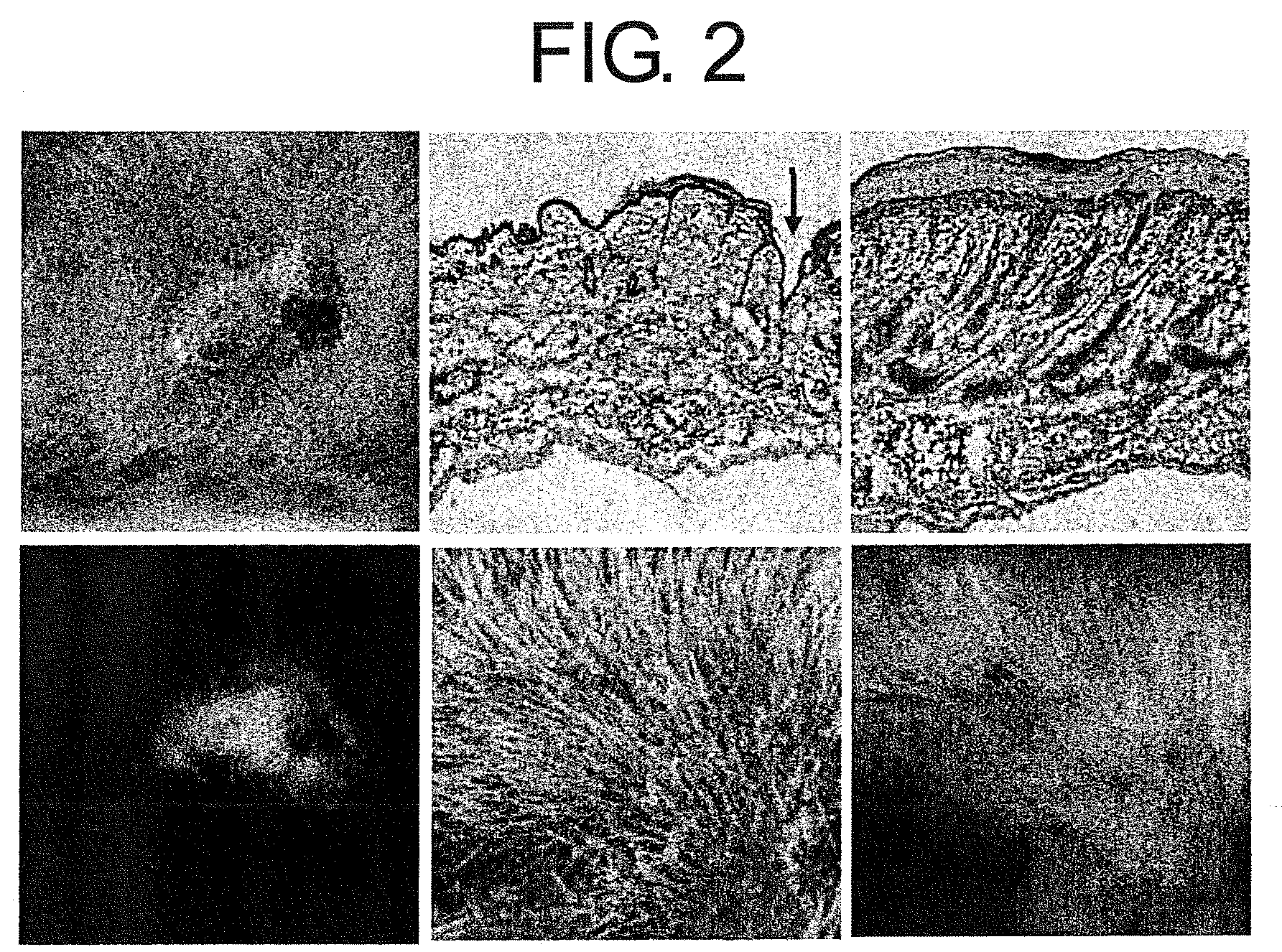
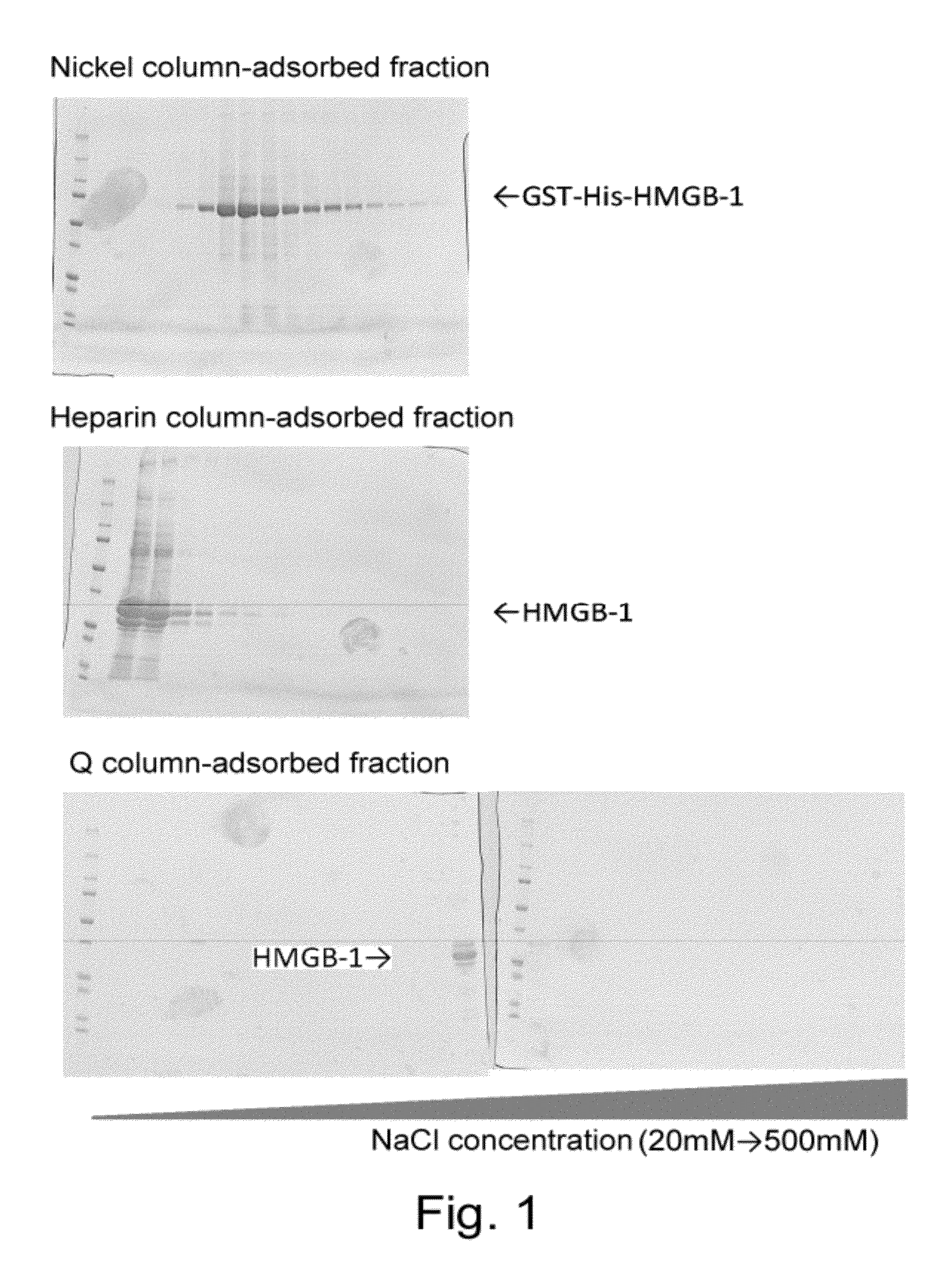
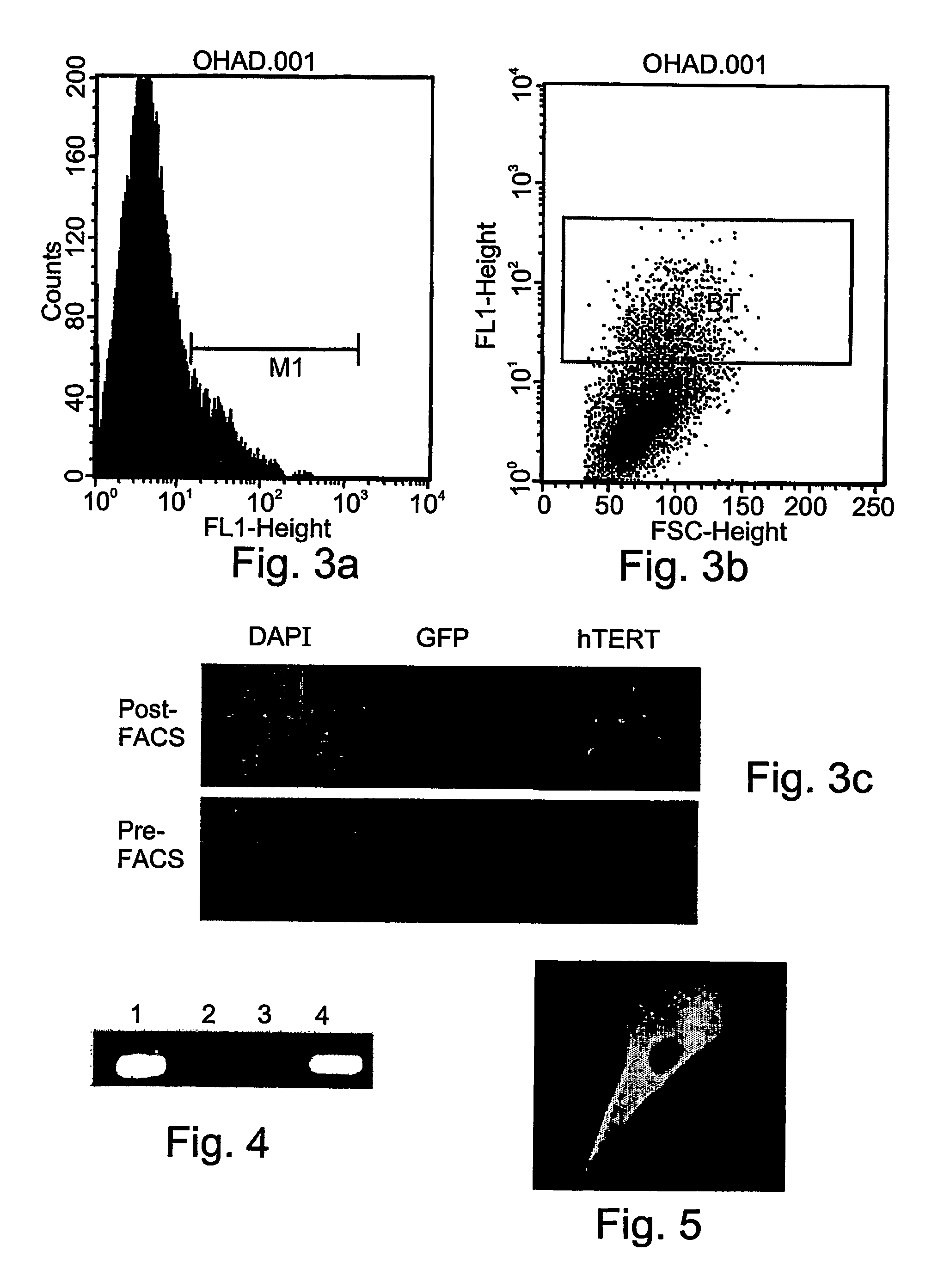
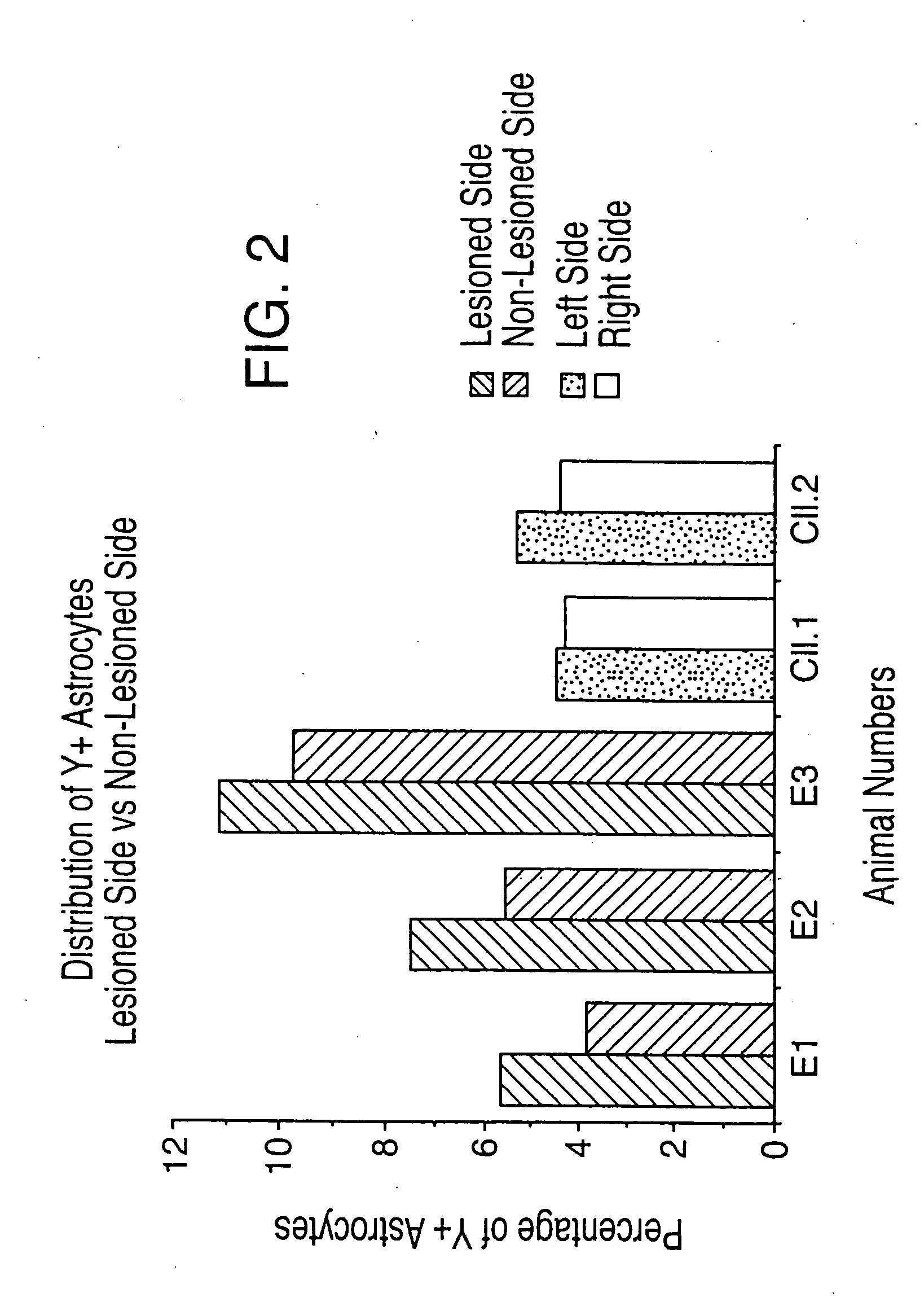
The present inventors revealed for the first time in the world that 1) a large amount of bone marrow-derived cells are mobilized in transplanted skin; 2) the mobilized bone marrow-derived cells are differentiated into any of dermal fibroblasts, adipocytes, muscle cells, vascular endothelial cells and epidermal keratinocytes in a transplanted skin graft, and in the mobilized bone marrow-derived cells, bone marrow-derived mesenchymal stem cells are contained; 3) what mobilize the bone marrow-derived mesenchymal stem cells to the graft from the peripheral blood are HMGB1, HMGB2 and HMGB3 released from necrotic tissue of the transplanted skin; 4) purified HMGB1, HMGB2 and HMGB3 promote migration of mesenchymal stem cells isolated from the bone marrow and cultured; 5) active substances including HMGB1 that allow bone marrow mesenchymal stem cells to migrate can be simply purified from an extract of a large number of organs including skin, brain and heart; 6) active substances that allow bone marrow mesenchymal stem cells to migrate can be simply extracted from cultured cells; and 7) a heparin column-purified fraction of skin extract mobilizes a large amount of bone marrow-derived cells during brain damage.
Owner:GENOMIX CO LTD +1
Pharmaceuticals That Promote Functional Regeneration of Damaged Tissues
InactiveUS20090202500A1Promote functional tissue regenerationPromote tissue regenerationOrganic active ingredientsPeptide/protein ingredientsDamages tissueInjury brain
The present inventors revealed the following for the first time in the world:1) a large amount of bone marrow-derived cells are mobilized to grafted skin;2) the mobilized bone marrow-derived cells are differentiated into any of dermal fibroblasts, adipocytes, muscle cells, vascular endothelial cells, and epidermal keratinocytes in the grafted skin, and the mobilized bone marrow derived cells include bone marrow-derived mesenchymal stem cells;3) the factors which mobilize bone marrow-derived mesenchymal stem cells from peripheral blood to the grafted skin are HMGB1, HMGB2, and HMGB3 released from the necrosed tissue of recipient skin;4) purified HMGB1, HMGB2, and HMGB3 promote the migration of mesenchymal stem cells isolated and cultured from bone marrow;5) activators containing HMGB1 which allows the migration of bone marrow mesenchymal stem cells can be conveniently purified from several organ extracts including skin, brain, and heart;6) activators which allow the migration of bone marrow mesenchymal stem cells can be conveniently extracted from cultured cells; and7) a heparin-column purified fraction of skin extract mobilizes a large amount of bone marrow-derived cells in case of brain injury.
Owner:OSAKA UNIV
Agents for promoting tissue regeneration by recruiting bone marrow mesenchymal stem cells and/or pluripotent stem cells into blood
ActiveUS20120251510A1Promote cell growthPromote functional regeneration/repairOrganic active ingredientsNervous disorderDamages tissueBone Marrow-Derived Cell
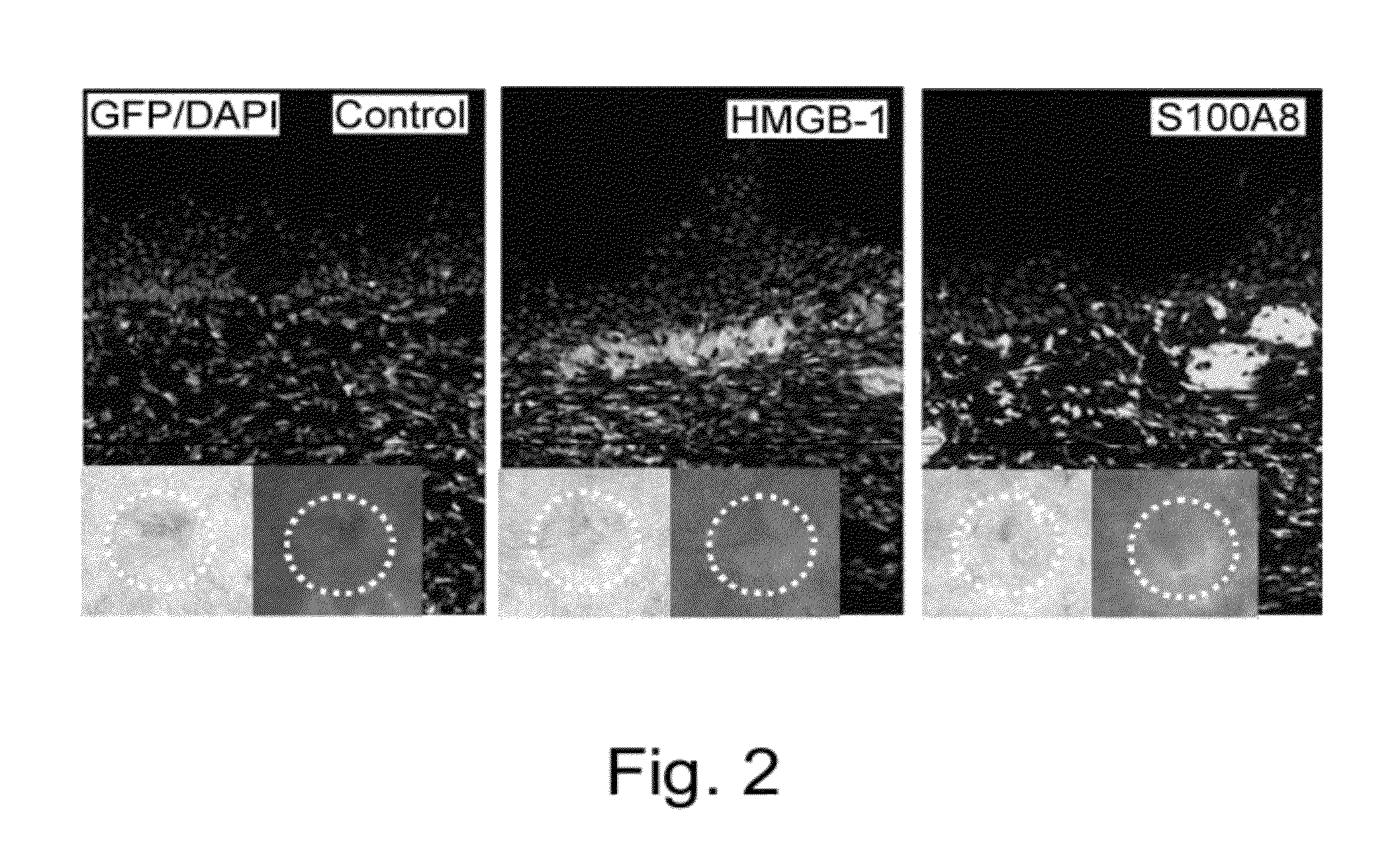
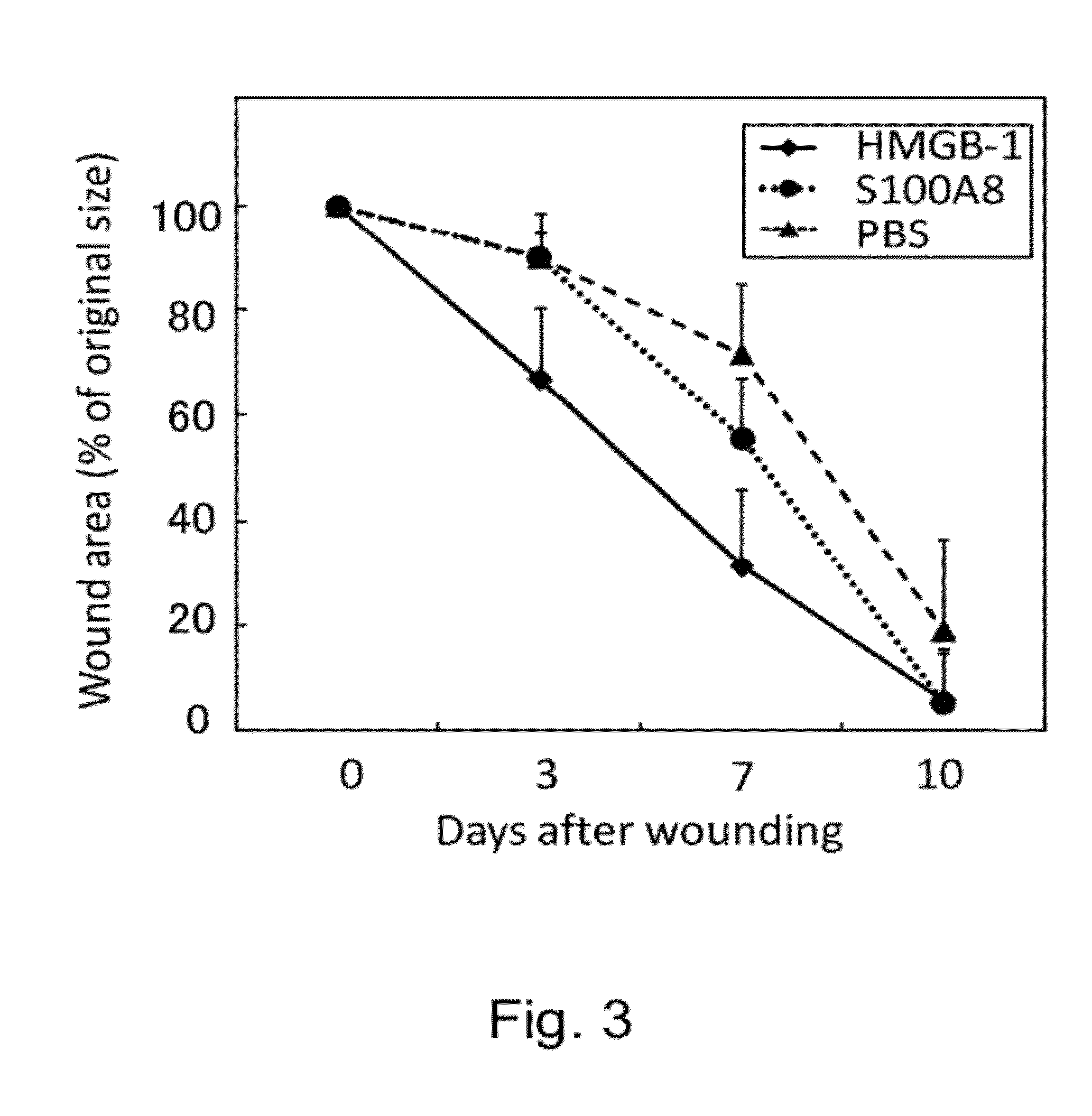
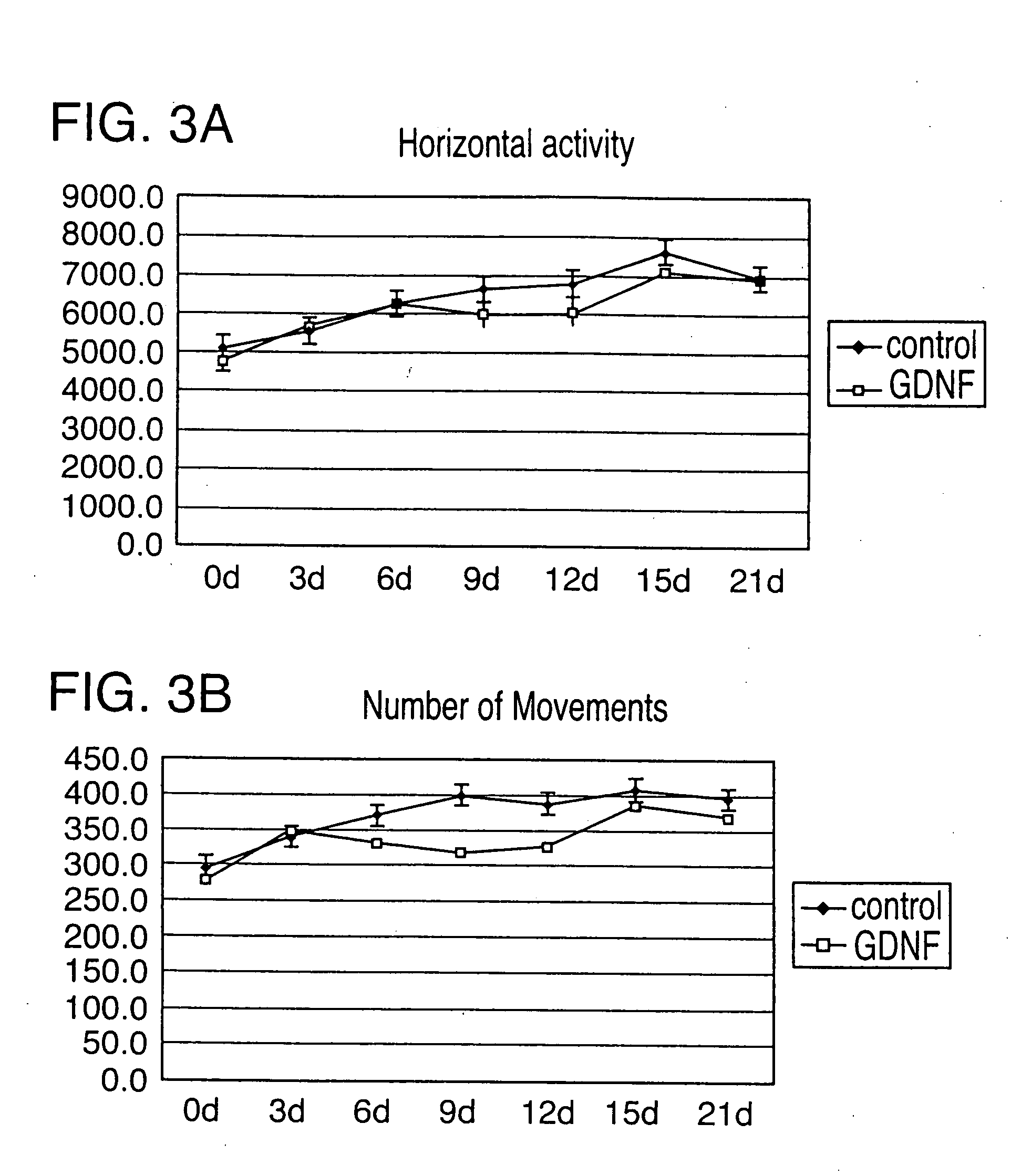
It was revealed that the intravenous administration of HMGB-1 and S100A8 promoted the healing of skin ulcer by recruiting bone marrow-derived cells to the site of skin ulcer. Furthermore, when HMGB-1 was intravenously administered to cerebral infarction model mice after creation of cerebral infarction, bone marrow-derived cells expressing nerve cell markers were detected in their brain. A marked cerebral infarct-reducing effect was observed in mice intravenously administered with HMGB-1 as compared to the control. The post-cerebral infarction survival rate was increased in the intravenous HMGB-1 administration group. The involvement of bone marrow pluripotent stem cells in the process of bone fracture healing was assessed using mice, and the result demonstrated that bone marrow-derived cells distant from the damaged site migrated to the bone fracture site to repair the damaged tissue.
Owner:STEMRIM INC +1
Pharmaceutical Agent for Promoting the Functional Regeneration of Damaged Tissue
InactiveUS20110097309A1Promote tissue regenerationOrganic active ingredientsBiocideDamages tissueVascular endothelium
The present inventors assessed the possibility that bone marrow-derived cells are mobilized to the grafted skin from nonskin tissues and contribute to skin tissue regeneration during the engraftment of grafted skin on biological tissues. As a result, the present inventors for the first time in the world demonstrated that:(1) a large number of bone marrow-derived cells are mobilized to grafted skin;(2) mobilized bone marrow-derived cells differentiate into any of dermal fibroblasts, adipocytes, muscle cells, vascular endothelial cells, and epidermal keratinocytes in grafted skin, and thus mobilized bone marrow-derived cells include bone marrow-derived mesenchymal stem cells;(3) S100A8 and S100A9 released from necrotic tissues of grafted skin are responsible for mobilizing bone marrow-derived mesenchymal stem cells to the grafted skin from peripheral blood; and(4) purified S100A8 and S100A9 promote the migration of mesenchymal stem cells isolated / cultured from the bone marrow.
Owner:OSAKA UNIV +1
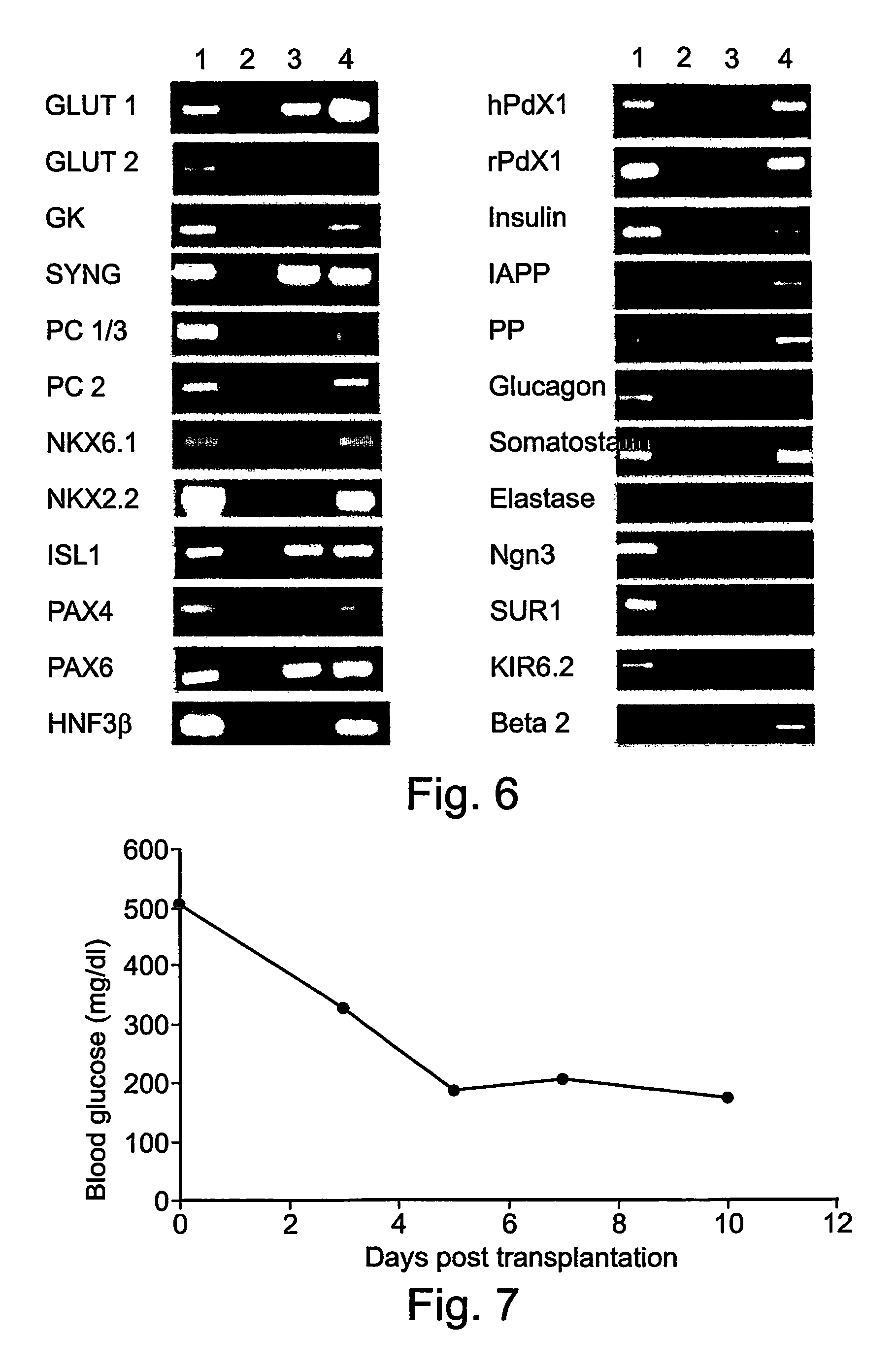
Insulin-producing bone marrow derived cells and methods of generating and using same
InactiveUS20060216277A1Lower blood sugar levelsBiocideGenetically modified cellsGlucose polymersD-Glucose
Insulin-producing bone marrow derived stem cells, and methods of generating and using same to reduce blood glucose levels in individuals.
Owner:RAMOT AT TEL AVIV UNIV LTD
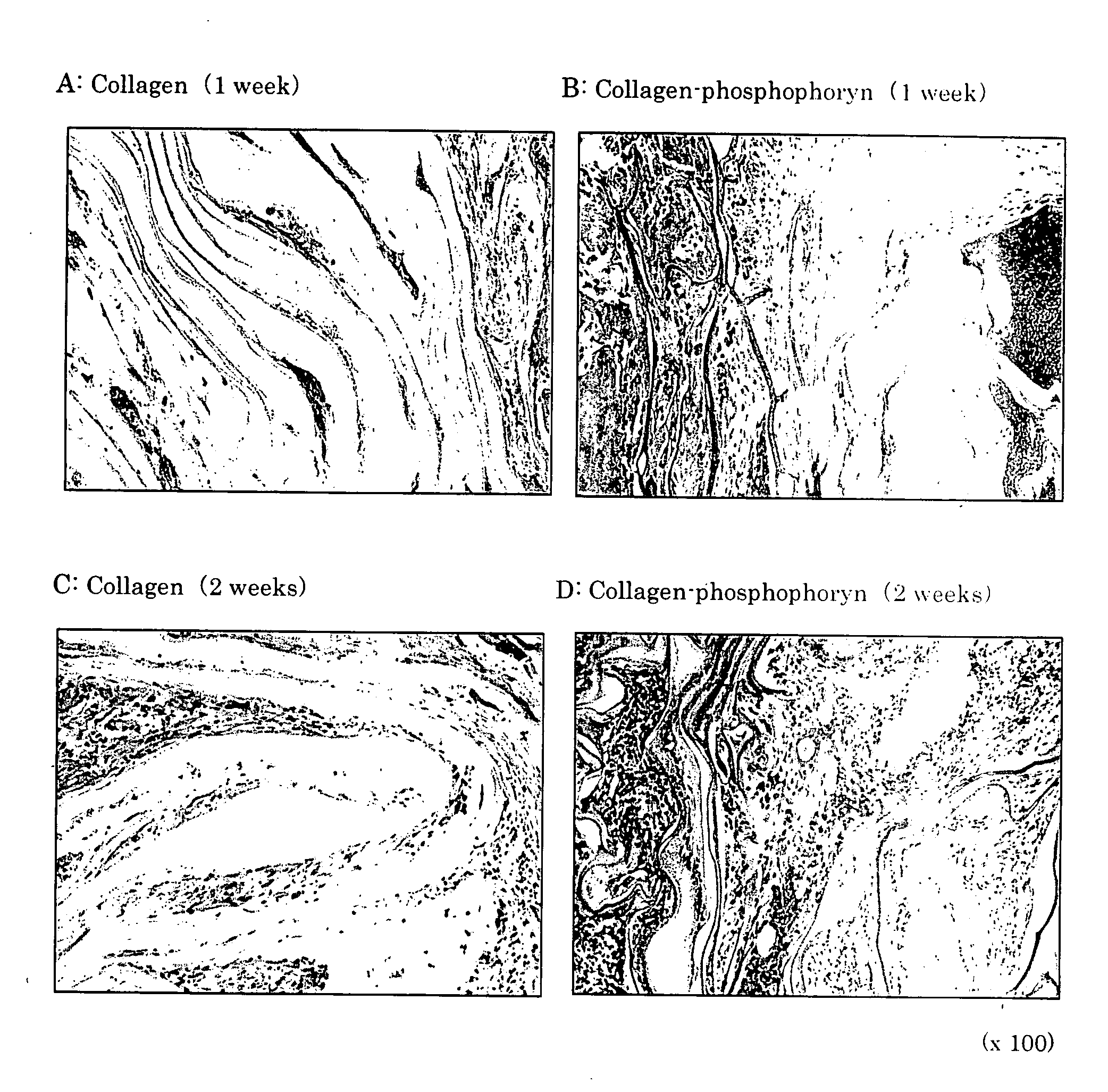
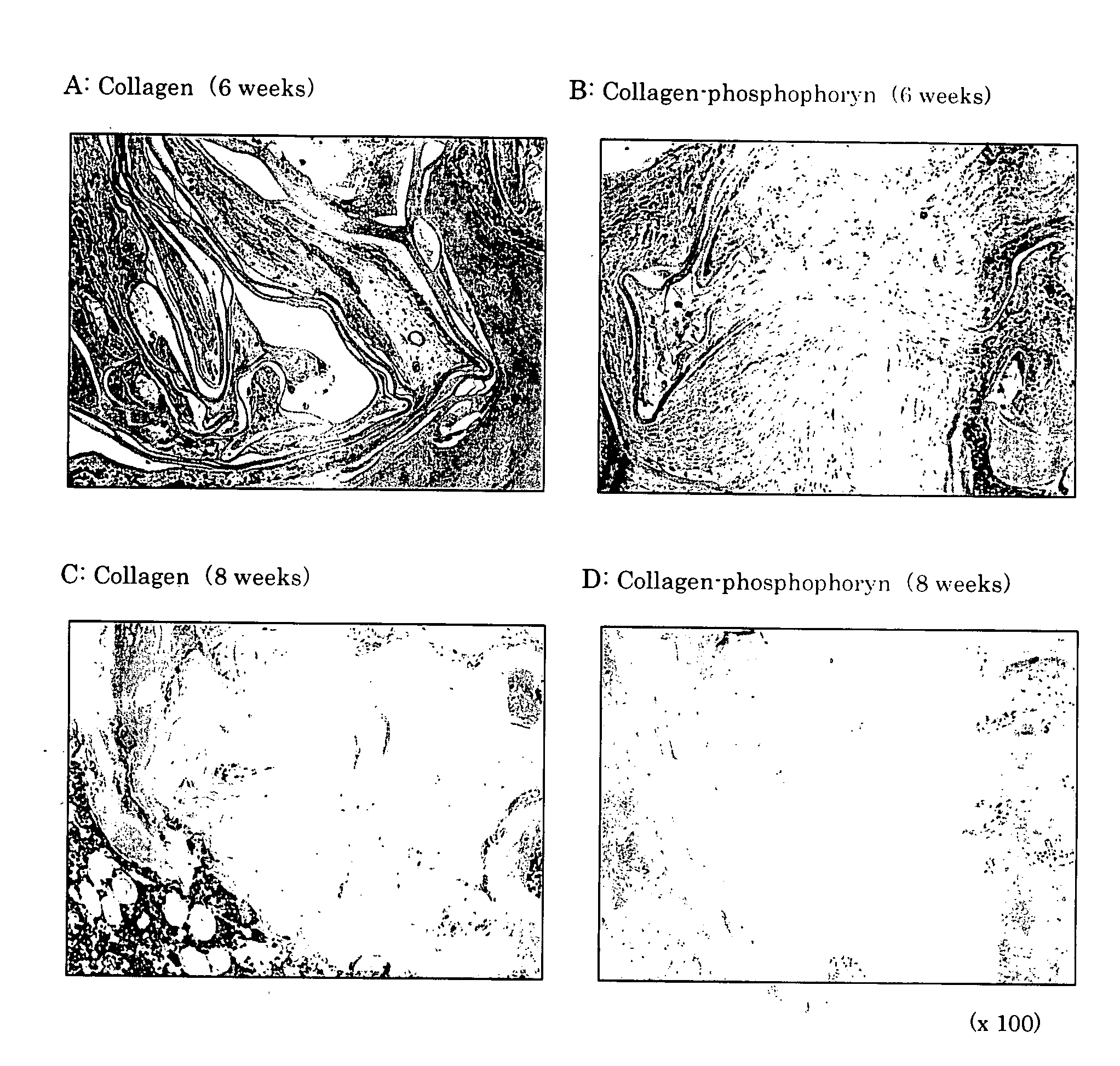
Composite biomaterial comprising phospholine
InactiveUS20050107286A1Enhanced osteogenic propertiesEasy to shapeBiocidePeptide/protein ingredientsBone Marrow-Derived CellArtificial bone
This invention relates to composite biomaterials having a sponge-like structure and comprising phosphophoryn and collagen, and to artificial bones obtained by culturing bone marrow-derived cells on the aforementioned composite biomaterials.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Preparation method of medical cartilage support material
ActiveCN103301508ASame mechanical strengthImprove efficiencyProsthesisPorosityBone Marrow-Derived Cell
The invention provides a preparation method of a medical cartilage support material. The preparation method comprises the following operation steps of: pretreatment, separation and primary washing of a cartilage tissue, inactivation of virus, decellularization, sodium chloride treatment, forming, and packaging and sterilization. The bone marrow-derived cell component and DNA (Deoxyribonucleic Acid) component of an animal are completely removed from the medical cartilage support material prepared by the method, meanwhile a natural ECM (Extracellular Cell Matrix) component and a three-dimensional are completely remained, and no endotoxin, organic solvent and toxic solvent are left; the medical cartilage support material has a porosity suitable for cell ingrowth and cartilage tissue regeneration, and also has ideal mechanical properties.
Owner:BEIJING BIOSIS HEALING BIOLOGICAL TECH
Method of regenerating bone/chondral tissues by transferring transcriptional factor gene
InactiveUS20050063959A1Improve compatibilityImprove bone formationBiocideGenetic material ingredientsBone Marrow-Derived CellCell biology
This invention provides a method of rapid and adequate culture of cells isolated from a body to effectively construct bone / cartilage tissues and implants containing the bone / cartilage tissues constructed by the aforementioned method. In this method, osteo- / chondro-inducible transcription factor genes are transfected into bone-marrow-derived cells isolated from a body using an adenoviral or a retroviral vector to grow on adequate scaffolds. The constructed bone / cartilage tissues are transplanted into a body together with the scaffolds. Thus, they can be used as substitutional bone / cartilage implants.
Owner:NAT INST OF ADVANCED IND SCI & TECH +1
Use of marrow-derived glial progenitor cells as gene delivery vehicles into the central nervous system
The present disclosure relates to a method for introducing a hematopoietic cell into the brain of a mammal, by administering bone marrow-derived progenitor cells into the body of the mammal by intravenous injection. The bone marrow-derived cell is preferably a cell that differentiates into a glial cell.The disclosure also relates to a method for delivery of therapeutic protein molecules into the brain of a mammal, by administering to a mammal an effective amount of bone marrow-derived progenitor cells which contain a gene having a nucleic acid sequence that encodes a functional therapeutic protein.Isolated recombinant cells and a pharmaceutical composition are also provided.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Pharmaceutical agent for promoting functional regeneration of damaged tissue
InactiveCN102076351AOrganic active ingredientsPeptide/protein ingredientsDamages tissueVascular endothelium
Studies have been made on a possibility that a bone-marrow-derived cell is recruited from an extracutaneous tissue into a skin graft during the process of adhesion of the skin graft to a biological tissue and therefore contributes to the regeneration of a skin tissue. As a result, the following facts 1) to 4) are found: 1) a large quantity of bone-marrow-derived cells are recruited into a skin graft; 2) the recruited bone-marrow-derived cells are differentiated into all of dermal fibroblasts, adipocytes, muscle cells, vascular endothelial cells and epidermal keratinocytes in the skin graft, and bone-marrow-derived mesenchymal stem cells are contained in the recruited bone-marrow-derived cells; 3) the substances which recruit the bone-marrow-derived mesenchymal stem cells from the peripheral blood into the skin graft are S100A8 and S100A9 which are released from a dead tissue in the skin graft; and 4) purified S100A8 and S100A9 can promote the migration of a mesenchymal stem cell which has been separated from a bone marrow and cultured.
Owner:GENOMIX CO LTD +1
Implant containing cells having growhfactor gene transferred thereinto
InactiveUS20050220773A1Improve biocompatibilityRapid bone regenerationBiocidePeptide/protein ingredientsGrowth Factor GeneBone implant
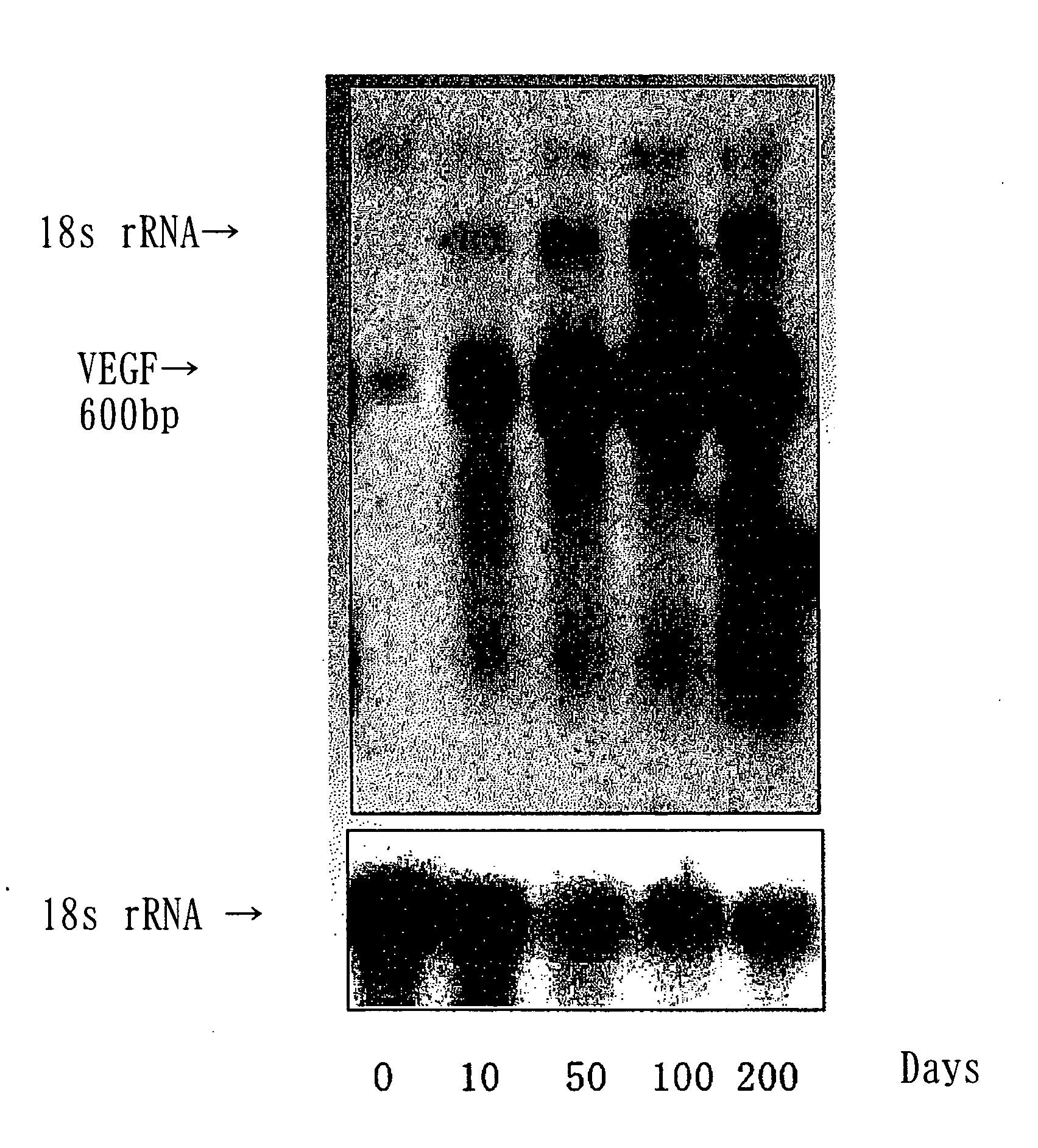
This invention provides alternative bone implants that have high bioadaptability and enable rapid bone regeneration. Specifically, this invention relates to implants consisting of a bioadaptable material comprising cells transfected with growth factor genes. Such implants are produced by inoculating bone-marrow-derived cells transfected with the genes of vascular endothelial growth factors (VEGF) into a bioadaptable material, such as porous ceramics, and culturing them.
Owner:NAT INST OF ADVANCED IND SCI & TECH +1
Use of vascular endothelial growth factor receptor 1+ cells in treating and monitoring cancer and in screening for chemotherapeutics
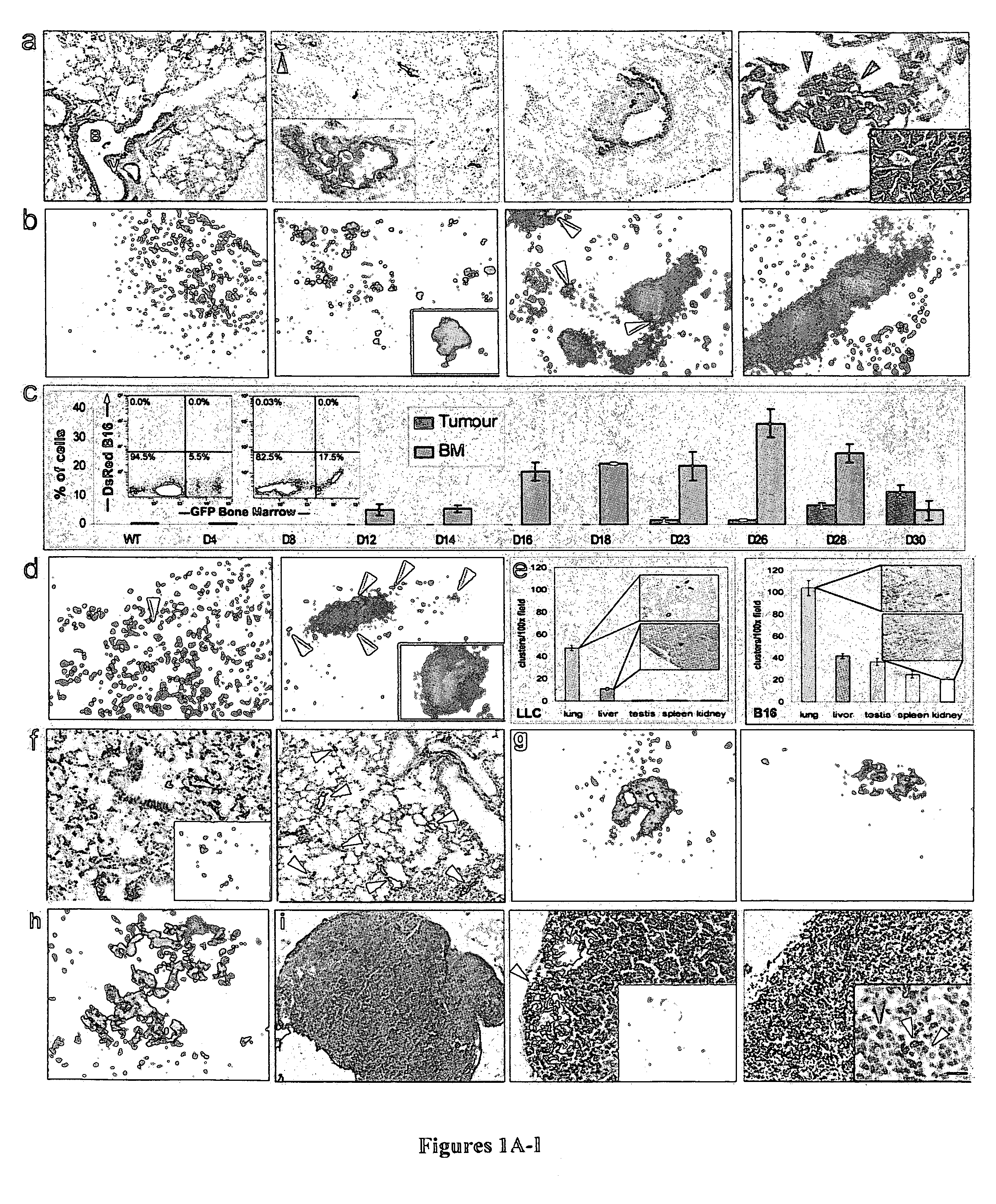
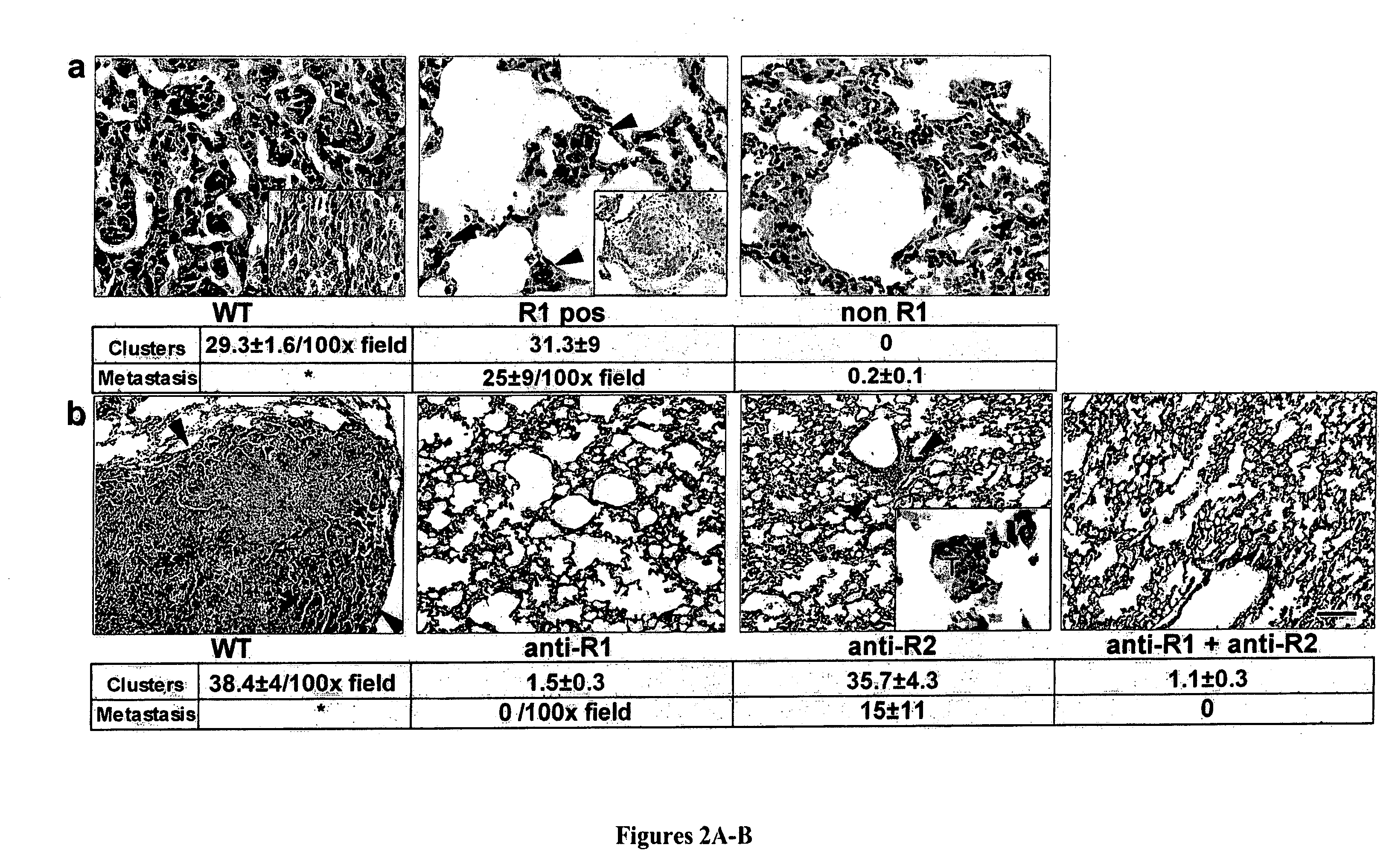
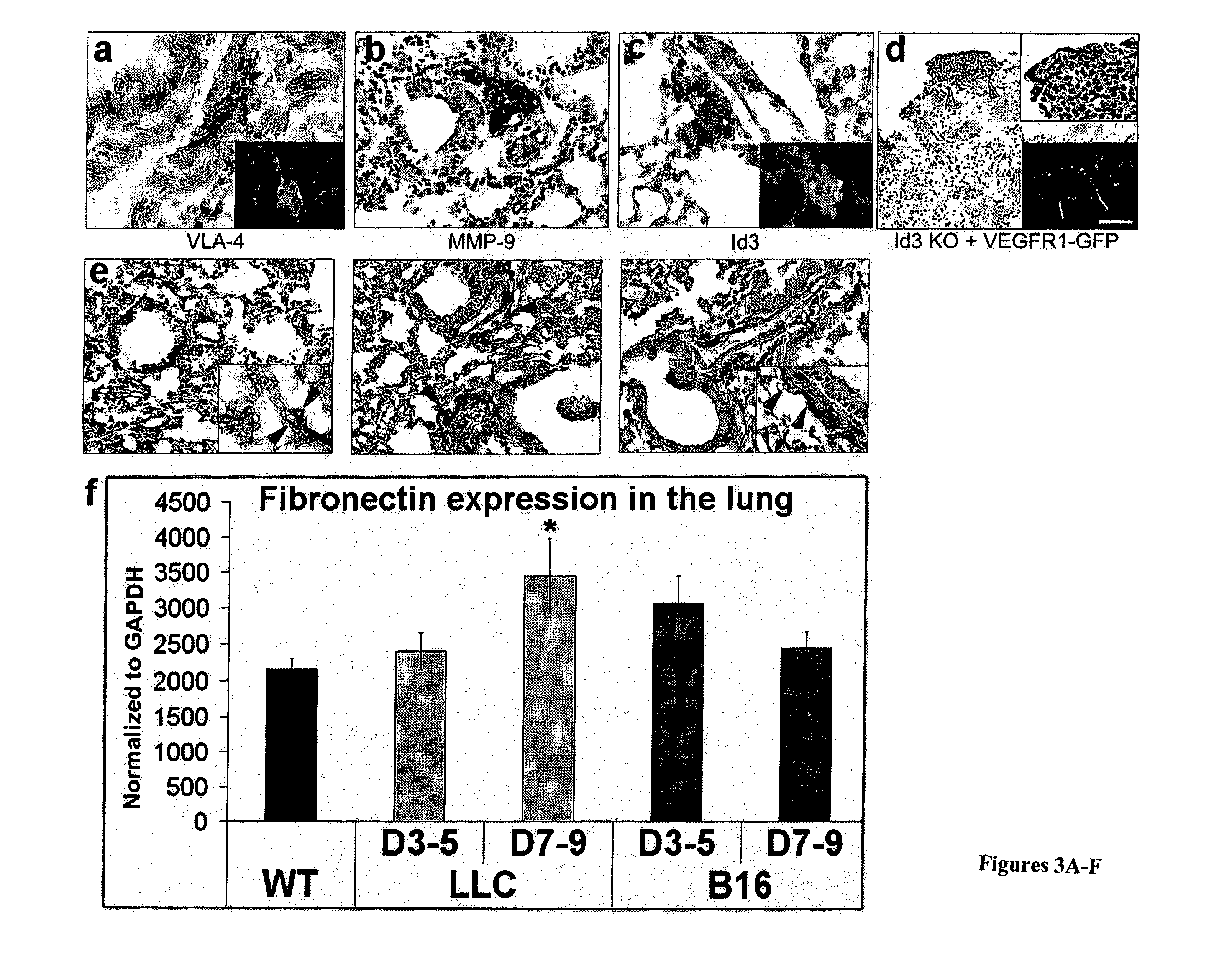
ActiveUS20100150932A1Care can be usedPromote migrationBiological material analysisAntibody ingredientsLymphatic SpreadBone Marrow-Derived Cell
The present invention is directed to a method of inhibiting tumor formation in a cancer patient at a site remote from sites of prior tumor formation and to a method of preventing metastases. These methods involve administering to the cancer patient an inhibitor of vascular endothelial growth factor receptor 1+ bone marrow-derived cells under conditions effective either to inhibit tumor formation in the cancer patient at a site remote from sites of prior tumor formation or to prevent metastases. Candidate compounds useful for such purposes can be screened depending on whether they bind to vascular endothelial growth factor receptor 1+ bone marrow-derived cells. Metastases in a cancer patient can be monitored by evaluating a patient sample for detection and quantification of vascular endothelial growth factor receptor 1+ bone marrow-derived cells and comparing the level of vascular endothelial growth factor receptor 1+ bone marrow-derived cells to prior levels.
Owner:CORNELL RES FOUNDATION INC
Stably transformed bone marrow-derived cells and uses thereof
InactiveUS20100028312A1Increased proliferationReduce apoptosisBiocidePeptide/protein ingredientsBone marrow cellBone Marrow-Derived Cell
The invention provides compositions comprising genetically modified bone marrow cells and related therapeutic and diagnostic methods. Transduced bone marrow cells can be therapeutically administered to a subject, such as a human patient to provide for the expression of an encoded protein in the subject in need thereof.
Owner:STEWARD RES & SPECIALTY PROJECTS
Use of marrow-derived glial progenitor cells as gene delivery vehicles into the central nervous system
The present disclosure relates to a method for introducing a hematopoietic cell into the brain of a mammal, by administering bone marrow-derived progenitor cells into the body of the mammal by intravenous injection. The bone marrow-derived cell is preferably a cell that differentiates into a glial cell. The disclosure also relates to a method for delivery of therapeutic protein molecules into the brain of a mammal, by administering to a mammal an effective amount of bone marrow-derived progenitor cells which contain a gene having a nucleic acid sequence that encodes a functional therapeutic protein. Isolated recombinant cells and a pharmaceutical composition are also provided.
Owner:UNITED STATES OF AMERICA
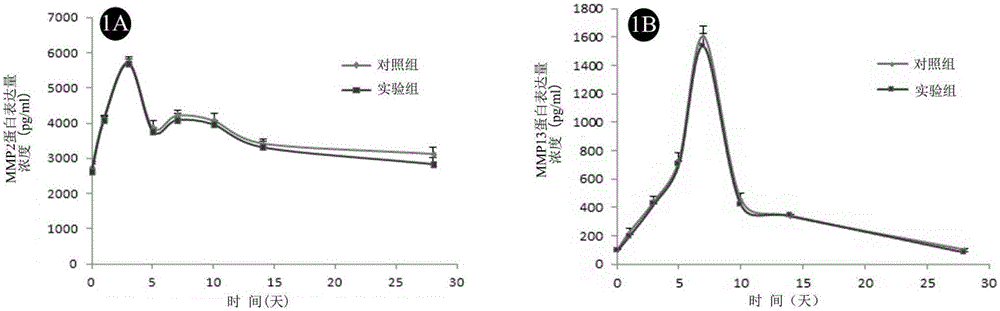
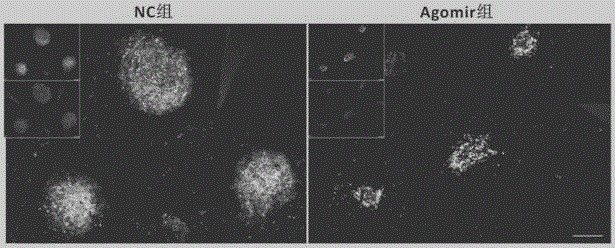
Application of micro RNA mimics in preparation of drug treating choroidal neovascularization
InactiveCN105126122AInhibition formationEffective treatmentOrganic active ingredientsSenses disorderFactor iiBone Marrow-Derived Cell
The invention provides application of micro RNA mimics in the preparation of a drug treating choroidal neovascularization (CNV). The inventor adjusts bone marrow derived cells MMP expression through micro RNA, utilizes the characteristic of chemotaxis of the bone marrow derived cells to CNV to realize high precision targeted drug delivery and sustained release drug delivery for a part of the CNV part, so as to inhibit the bone marrow derived cells from forming pathological new vessels together with in situ cells. The invention also provides application of micro RNA agomir in the preparation of a drug treating the choroidal neovascularization. The inventor simultaneously adjusts two types of MMP expressions of BMCS through the micro RNA agomir, so the invasion of the BMCS is inhibited to reduce CNV cells and factor sources, and the in situ cells are inhibited from migrating, and the CNV can be efficiently treated; besides, due to the characteristic of the BMCS only enriching at a local part of the CNV, a high precision targeted therapy can be realized at the part of the CNV.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Method of testing myelotoxicity with the use of flow cytometer
The objects of the present invention are to solve low test efficiency and low accuracy which are problems encountered in the conventional methods for testing myelotoxicity of a drug by observing smears. To this end, markers for discriminating and identifying hematopoietic stem cells and blood cells at various differentiation stages in bone marrow are identified. From this point of view, cell surface antigens are specified. Thus, it is found that the myelotoxicity of a drug can be evaluated with a high efficiency at a high accuracy by using flow cytometry to analyze for a change in the quantity of bone marrow-derived cells expressing the cell surface antigens after administration of the drug to an animal.
Owner:TEIJIN LTD
Use of vascular endothelial growth factor receptor 1+ cells in treating and monitoring cancer and in screening for chemotherapeutics
InactiveCN101107011AImprove adhesionDead animal preservationAntibody ingredientsLymphatic SpreadBone Marrow-Derived Cell
The present invention is directed to a method of inhibiting tumor formation in a cancer patient at a site remote from sites of prior tumor formation and to a method of preventing metastases. These methods involve administering to the cancer patient an inhibitor of vascular endothelial growth factor receptor 1+ bone marrow-derived cells under conditions effective either to inhibit tumor formation in the cancer patient at a site remote from sites of prior tumor formation or to prevent metastases. Candidate compounds useful for such purposes can be screened depending on whether they bind to vascular endothelial growth factor receptor 1+ bone marrow-derived cells and comparing the level of vascular endothelial growth factor receptor 1+ bone marrow-derived cells to prior levels.
Owner:CORNELL RES FOUNDATION INC
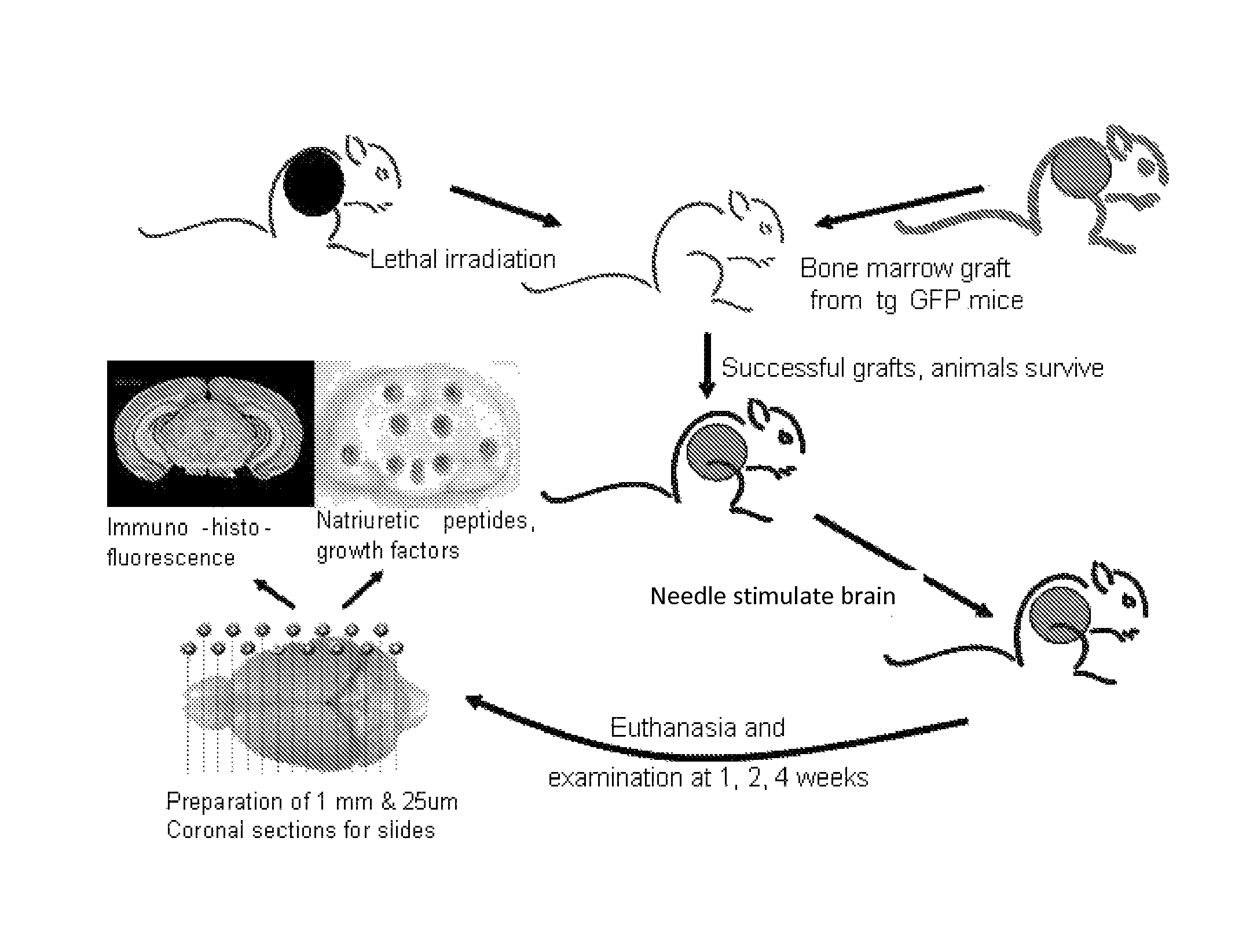
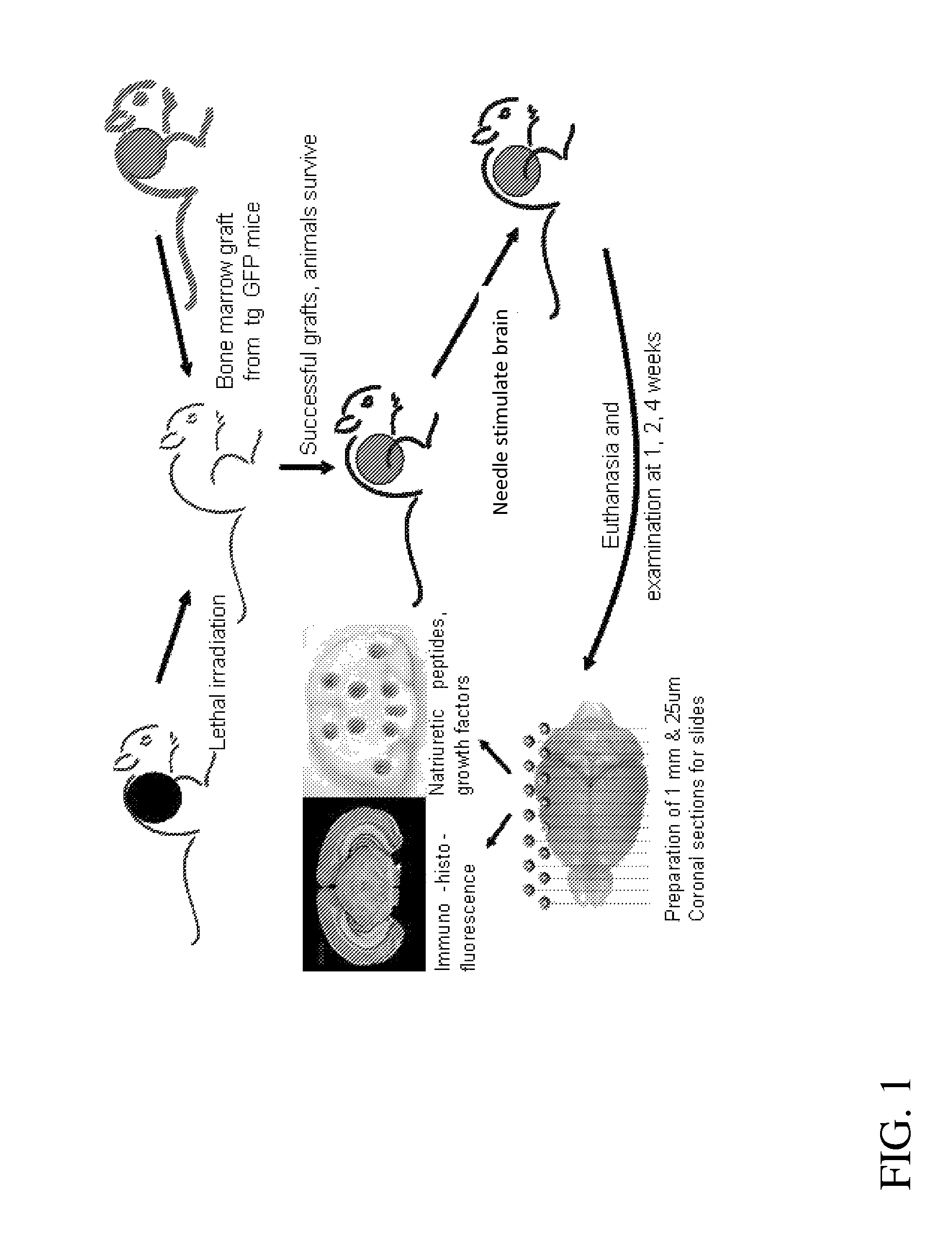
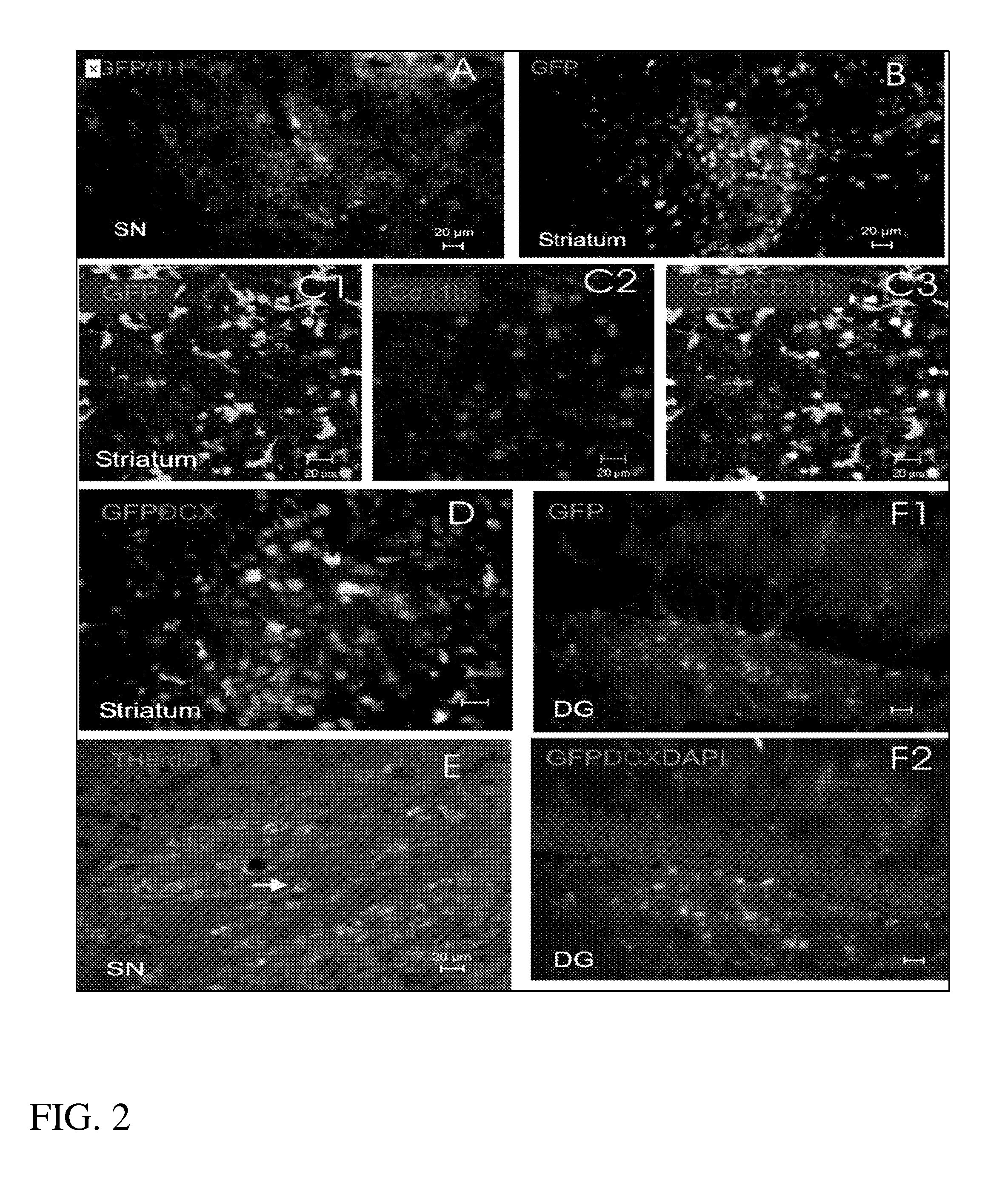
Promotion of brain self-repair mechanisms by stereotaxic micro-stimulation
Owner:UNIV OF SOUTH FLORIDA +1
Injection of bone marrow-derived cells and medium for angiogenesis
Methods are provided for enhancing capacity of impaired bone marrow cells to promote angiogenesis when introduced into an ischemic site in a patient by transfecting early attaching cells derived from bone marrow in culture with an angiogenesis promoting transgene. Methods are also provided for utilizing such early attaching cells derived from autologous bone marrow, or media derived from these cells while the cells are grown in culture (which need not be from autologous cells) to deliver angiogenesis-promoting transgenes or proteins to a patient. The transfected early attaching cells, or media derived from these cells while the cells are grown in culture, are introduced into an ischemic tissue, such as the heart, to enhance formation of collateral blood vessels. The cells or media can also be injected into the blood stream (artery supplying the ischemic tissue, or any other artery or vein).
Owner:MYOCARDIAL THERAPEUTICS INC
Methods for Regenerating and Repairing Damaged Tissues Using Adrenomedullin
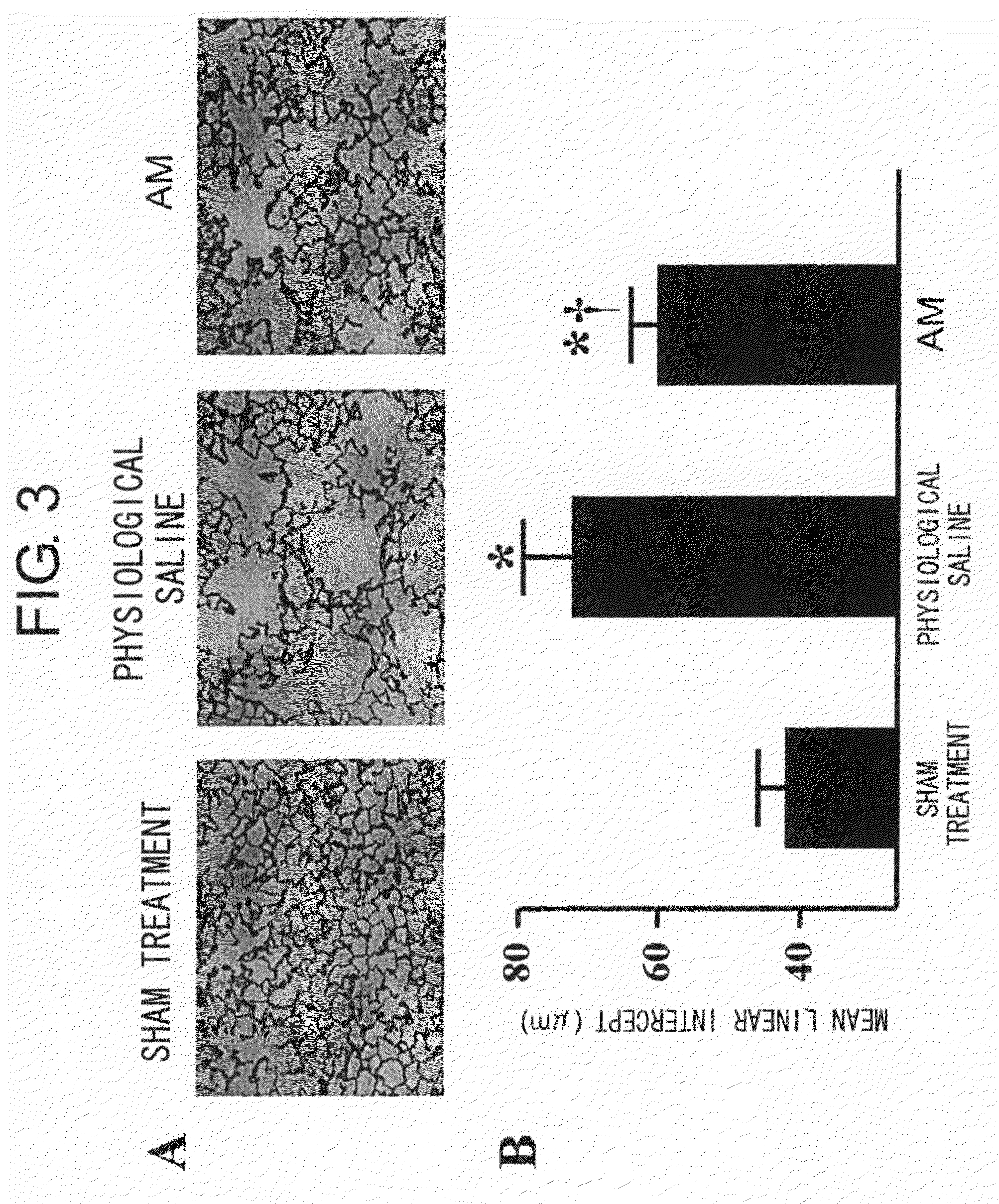
InactiveUS20090105171A1Increase the number ofIncreasing revascularizationOrganic active ingredientsPeptide/protein ingredientsAntigenDamages tissue
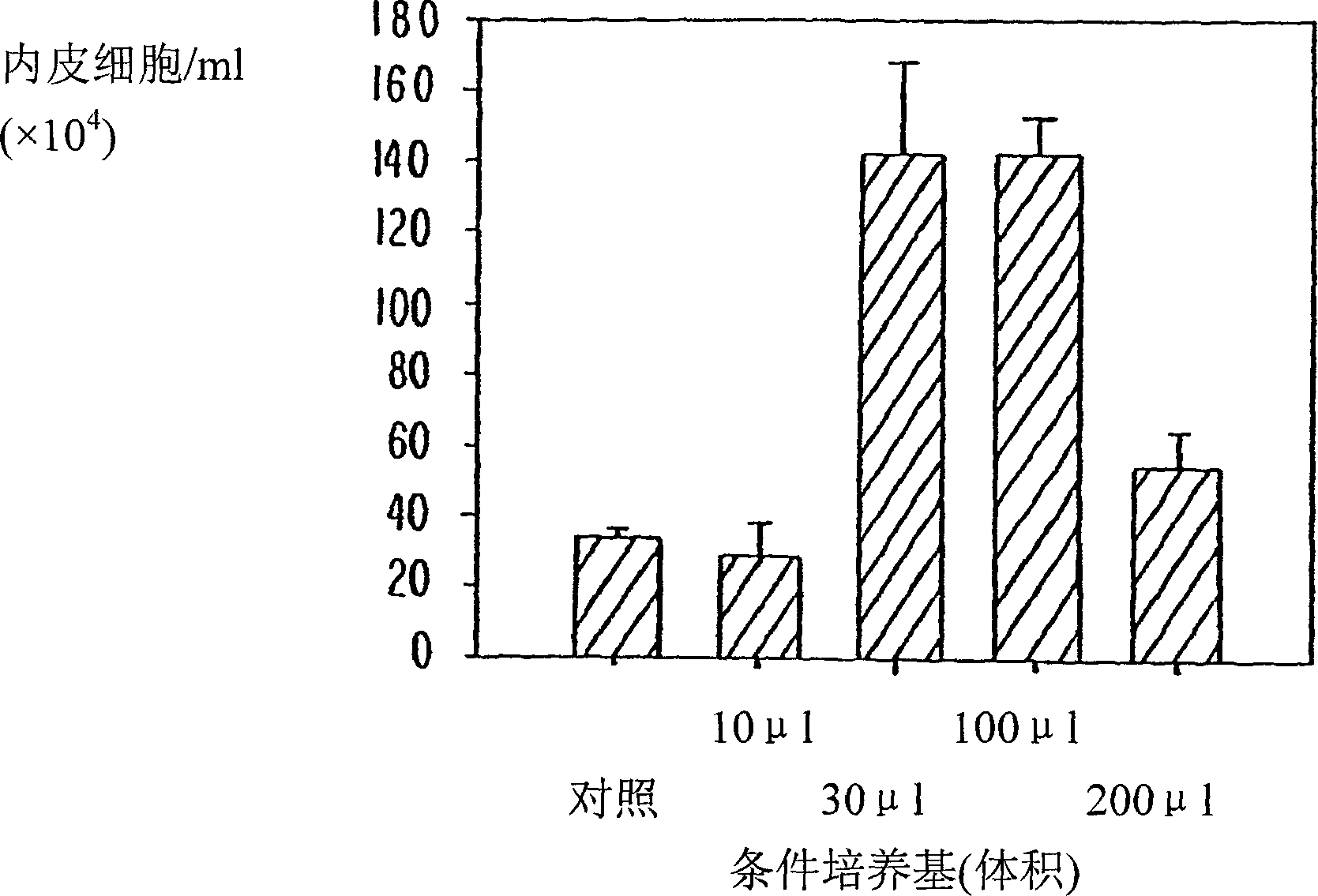
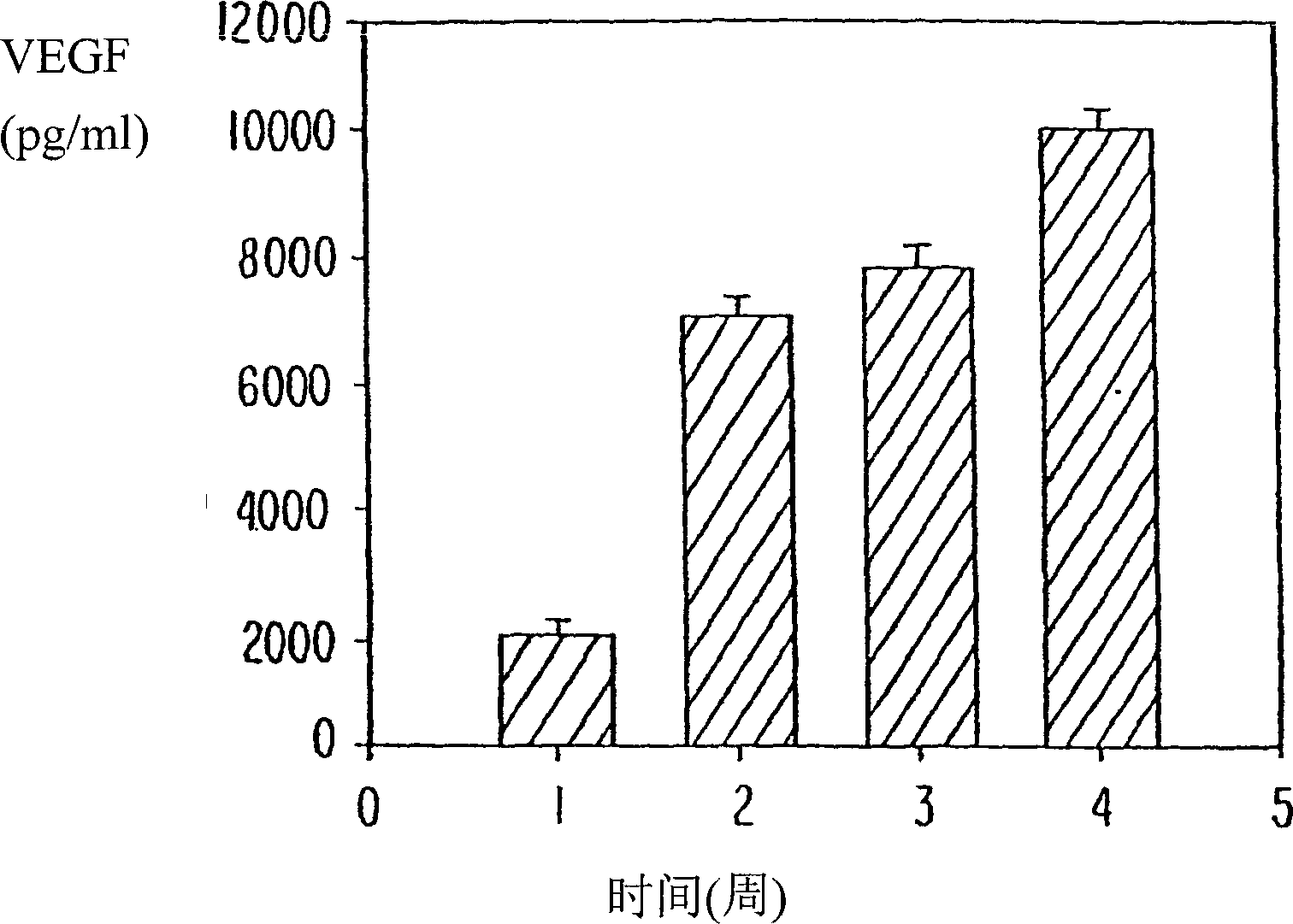
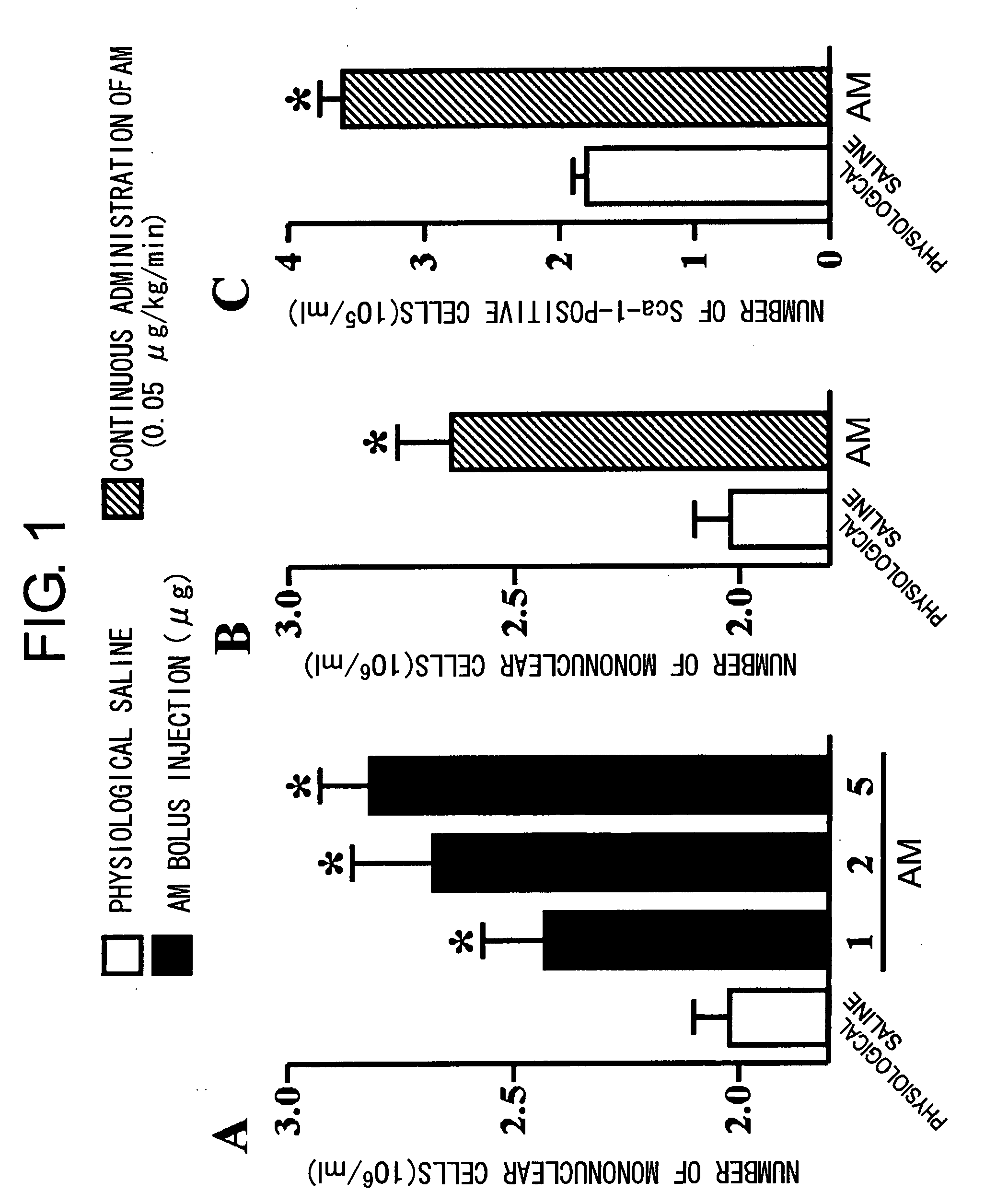
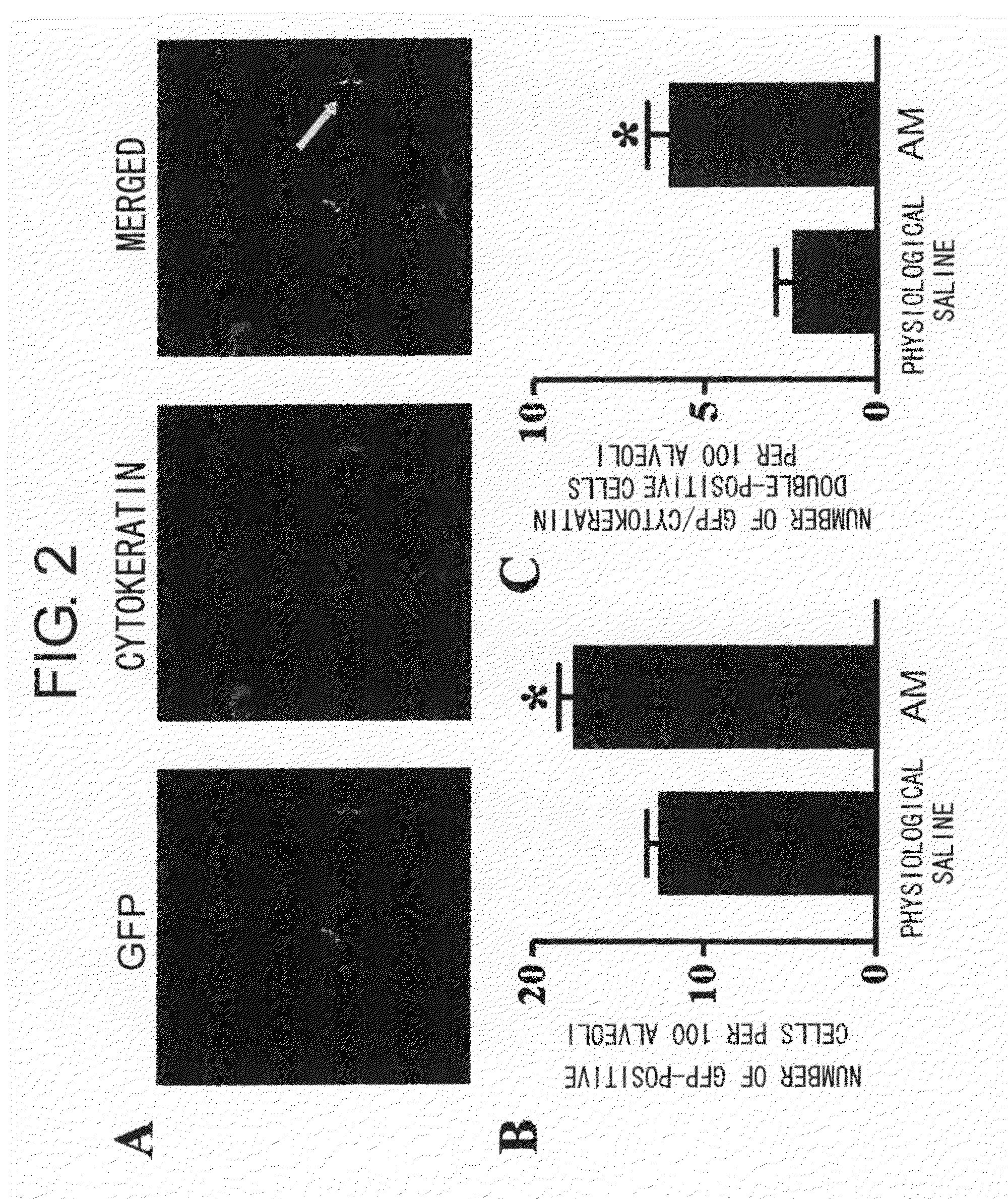
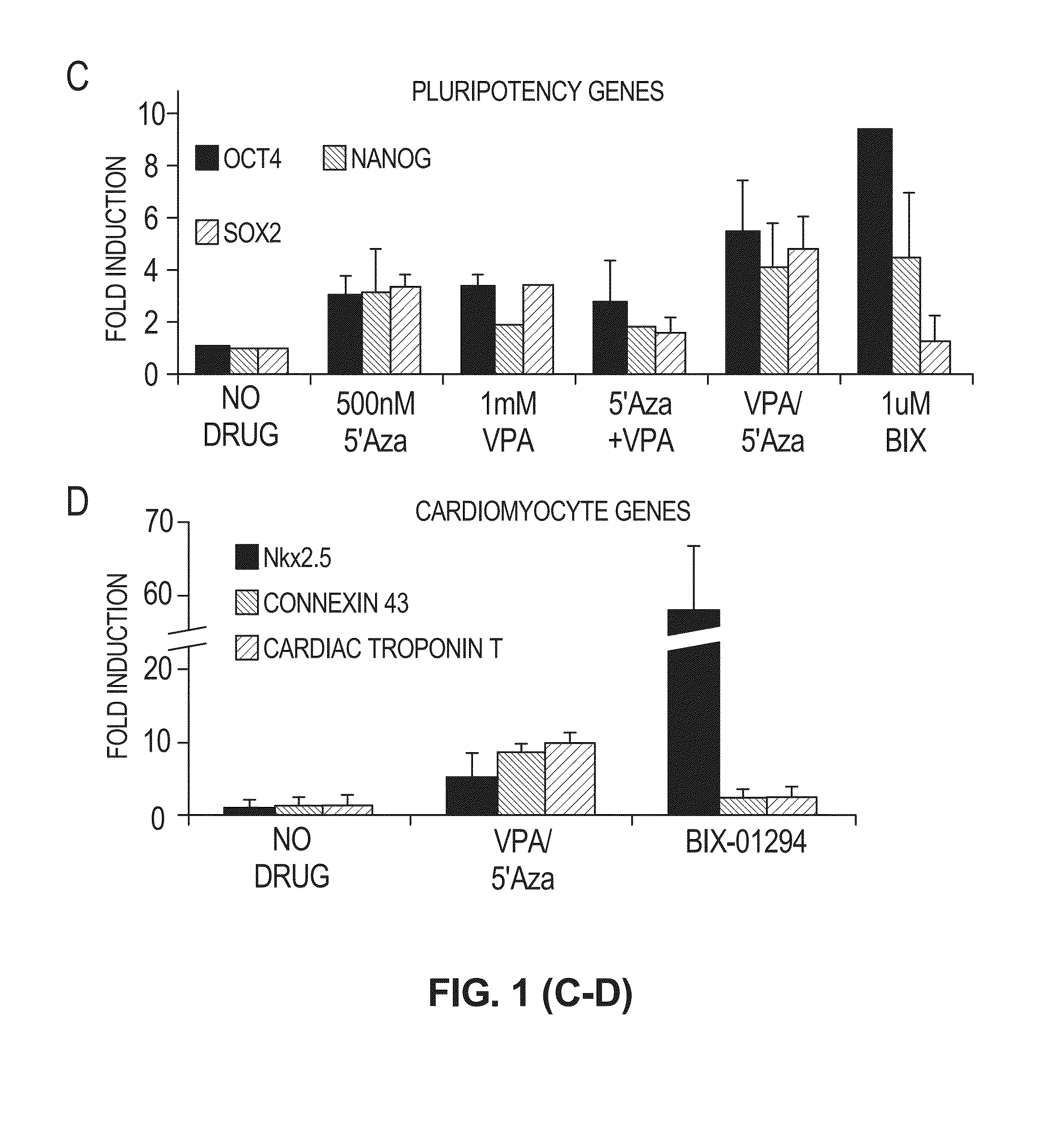
[Problems to be Solved] An objective of the present invention is to provide methods for regenerating or repairing damaged tissues using adrenomedullin. Another objective is to provide pharmaceutical agents that comprise adrenomedullin as an active ingredient for regenerating or repairing damaged tissues.[Means for Solving the Problems] To solve the above problems, the present inventors administered adrenomedullin (hereinafter indicated as AM) or physiological saline to C57BL / 6 mice, and counted the numbers of mononuclear cells and Sca-1-positive cells in the blood. The result showed that AM increased the numbers of mononuclear cells and stem cell antigen-1-positive cells in the blood. It was also shown that by administering AM to a mouse model of pulmonary emphysema and a rat model of acute myocardial infarction, the quantity of bone marrow-derived cells that migrated and settled into the damaged tissues increased, and the recruited bone marrow cells differentiated into blood vessels, alveoli, and cardiac muscle at the lesion sites. Further, decrease in infarct size, suppression of the enlargement of alveolar diameter, and improvement of organ functions were confirmed in the models of myocardial infarction and pulmonary emphysema.
Owner:HUBIT GENOMIX +1
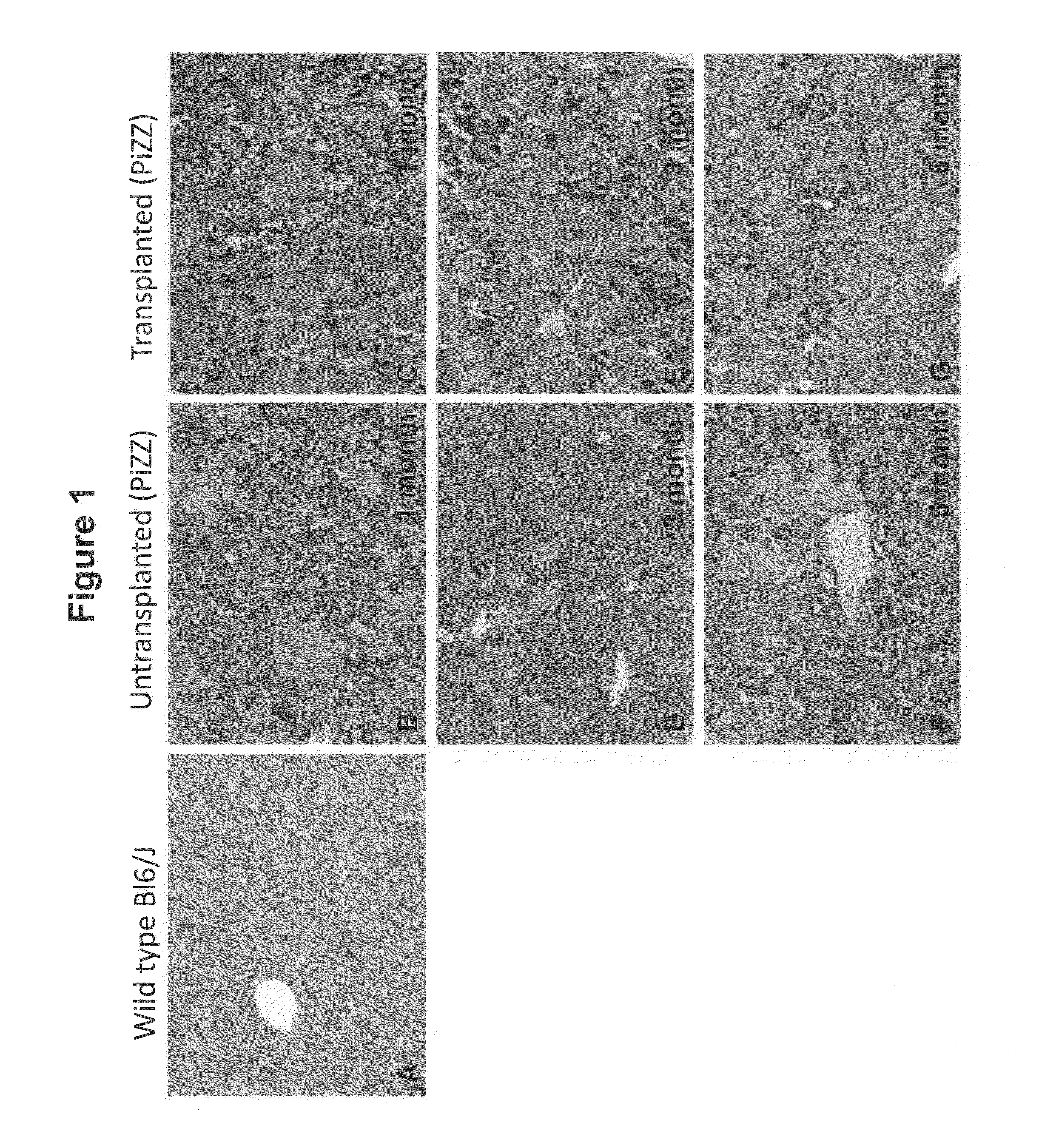
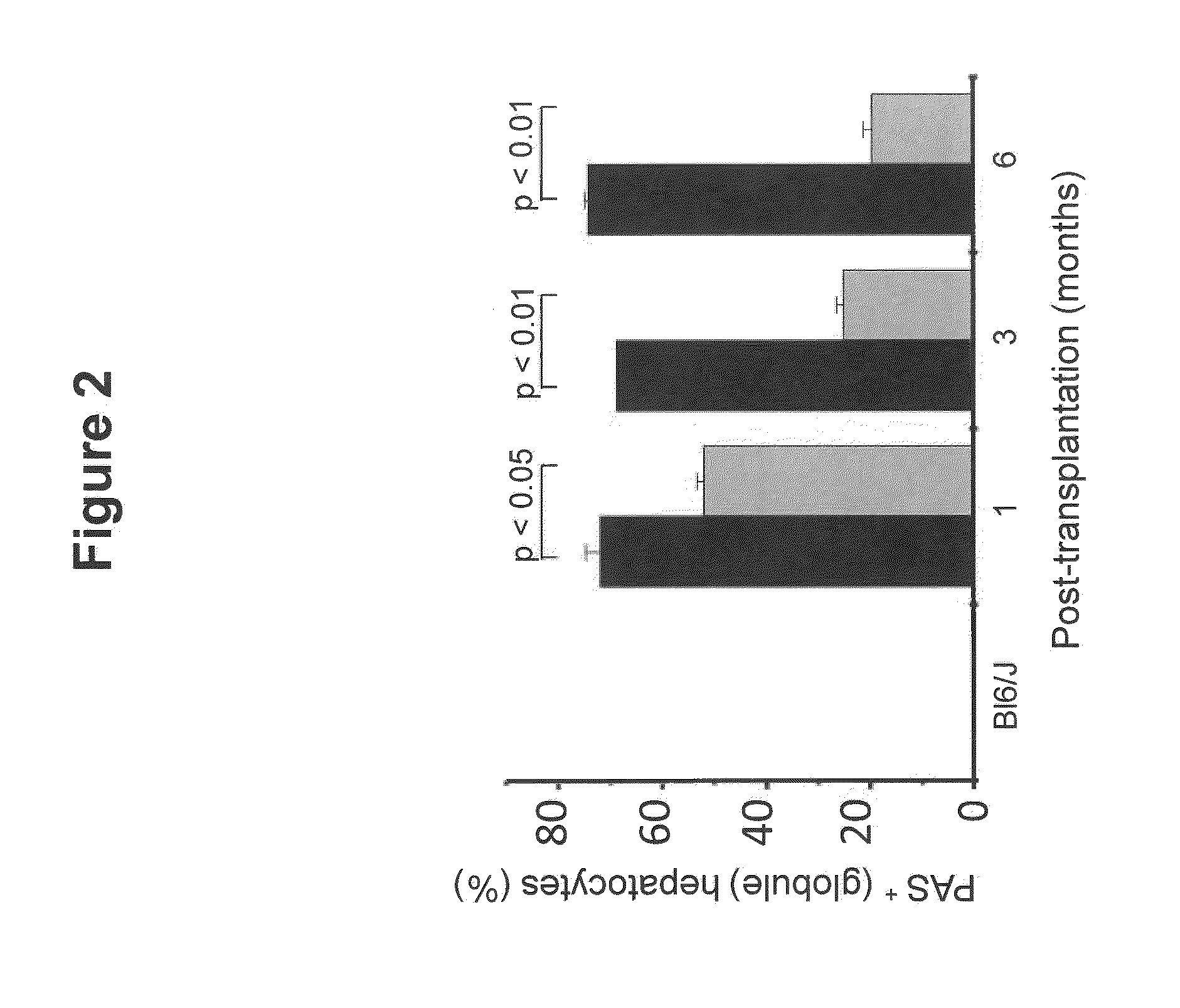
Bone Marrow-Derived Cells Ameliorates The Pathological Consequences Of The Liver In Case Of Alpha1-Antitrypsin Deficiency
The present invention is based on the findings that bone marrow (BM)-derived progenitor cells more specifically mesenchymal stem cells (MSCs), hematopoietic stem cells (HSCs) and uncommitted hematopoietic cells (lin−) are capable of regenerating liver in case of injury. The invention provides a method for treating genetic disorder like Alpha1-antitrypsin deficiency (A1-ATD) by administering BM derived Lin− cells in human mutant A1-AT expressing transgenic mouse model. The invention also provides the state of art for replacement of mutant host hepatocytes by transplanting wild-type uncommitted donor (lin−) cells.
Owner:NATIONAL INSTUTUTE OF IMMUNOLOGY
Use of vascular endothelial growth factor receptor 1+ cells in treating and monitoring cancer and in screening for chemotherapeutics
InactiveUS7598043B2Care can be usedPromote migrationBiological material analysisAntibody ingredientsLymphatic SpreadFactor ii
The present invention is directed to a method of inhibiting tumor formation in a cancer patient at a site remote from sites of prior tumor formation and to a method of preventing metastases. These methods involve administering to the cancer patient an inhibitor of vascular endothelial growth factor receptor 1+ bone marrow-derived cells under conditions effective either to inhibit tumor formation in the cancer patient at a site remote from sites of prior tumor formation or to prevent metastases. Candidate compounds useful for such purposes can be screened depending on whether they bind to vascular endothelial growth factor receptor 1+ bone marrow-derived cells. Metastases in a cancer patient can be monitored by evaluating a patient sample for detection and quantification of vascular endothelial growth factor receptor 1+ bone marrow-derived cells and comparing the level of vascular endothelial growth factor receptor 1+ bone marrow-derived cells to prior levels.
Owner:CORNELL RES FOUNDATION INC
Oligonucleotide compositions and their use to induce differentiation of cells
InactiveUS20070010472A1Augment net therapeutic effectOrganic active ingredientsSugar derivativesBone Marrow-Derived CellLeukopenia
The present invention provides compositions comprising a 3′-OH, 5′-OH, chemically unmodified, synthetic phosphodiester nucleotide sequence selected from the group consisting of SEQ ID NO: 1, SEQ ID NO: 2, SEQ ID NO: 3, and SEQ ID NO: 4, and a pharmaceutically acceptable carrier, wherein the compositions are useful to induce differentiation of cells or to stimulate differentiation or production of pluripotent cells. The present invention provides methods of using these compositions to induce differentiation of pluripotent cells, including bone marrow derived cells, and to treat disease associated with insufficient differentiation of cells in animals and humans, including but not limited to leukemia, lymphoma, non-malignant blood disorders such as hemoglobinopathies, sickle cell disease, myelodysplastic syndrome, pancytopenia, anemia, thrombocytopenia and leukopenia.
Owner:BIONICHE LIFE SCI
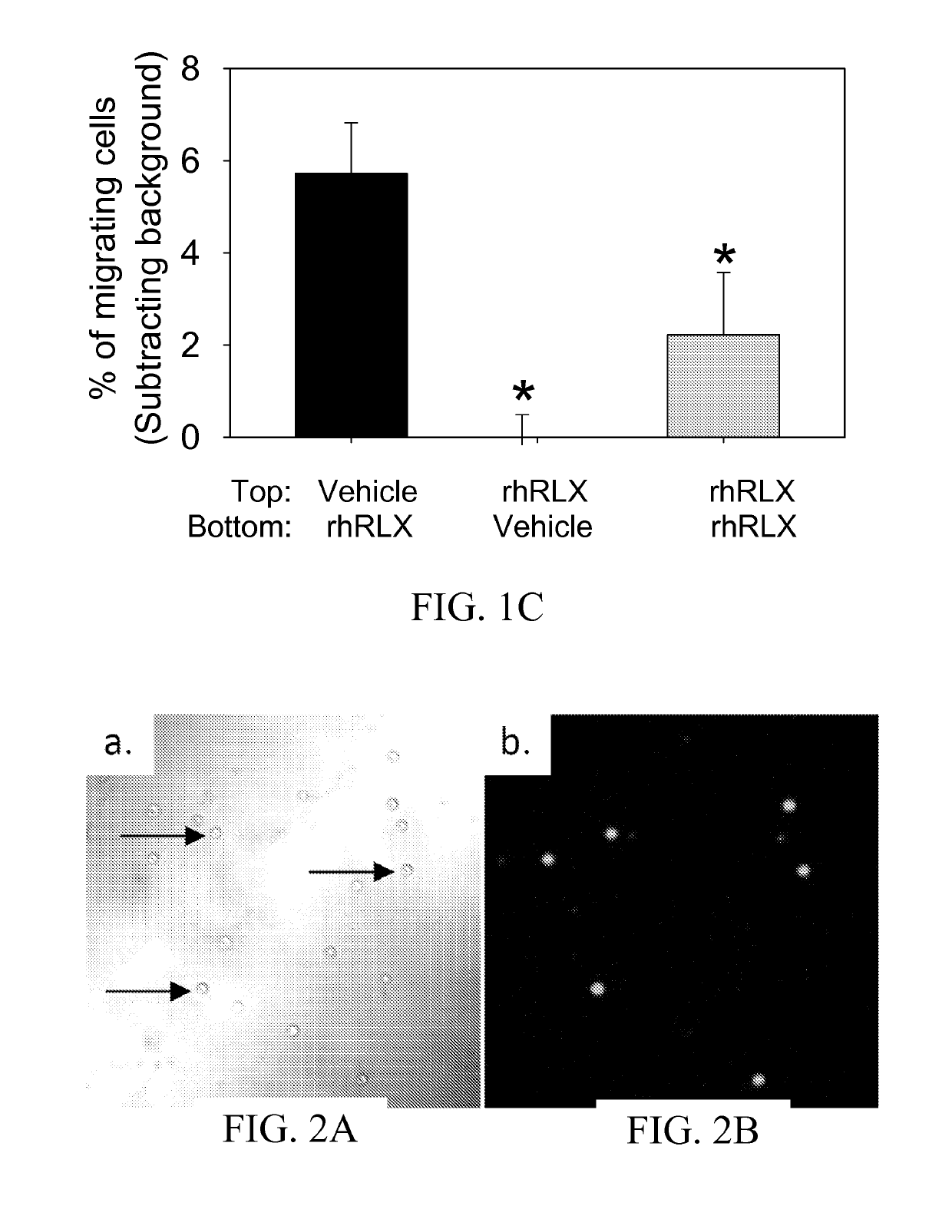
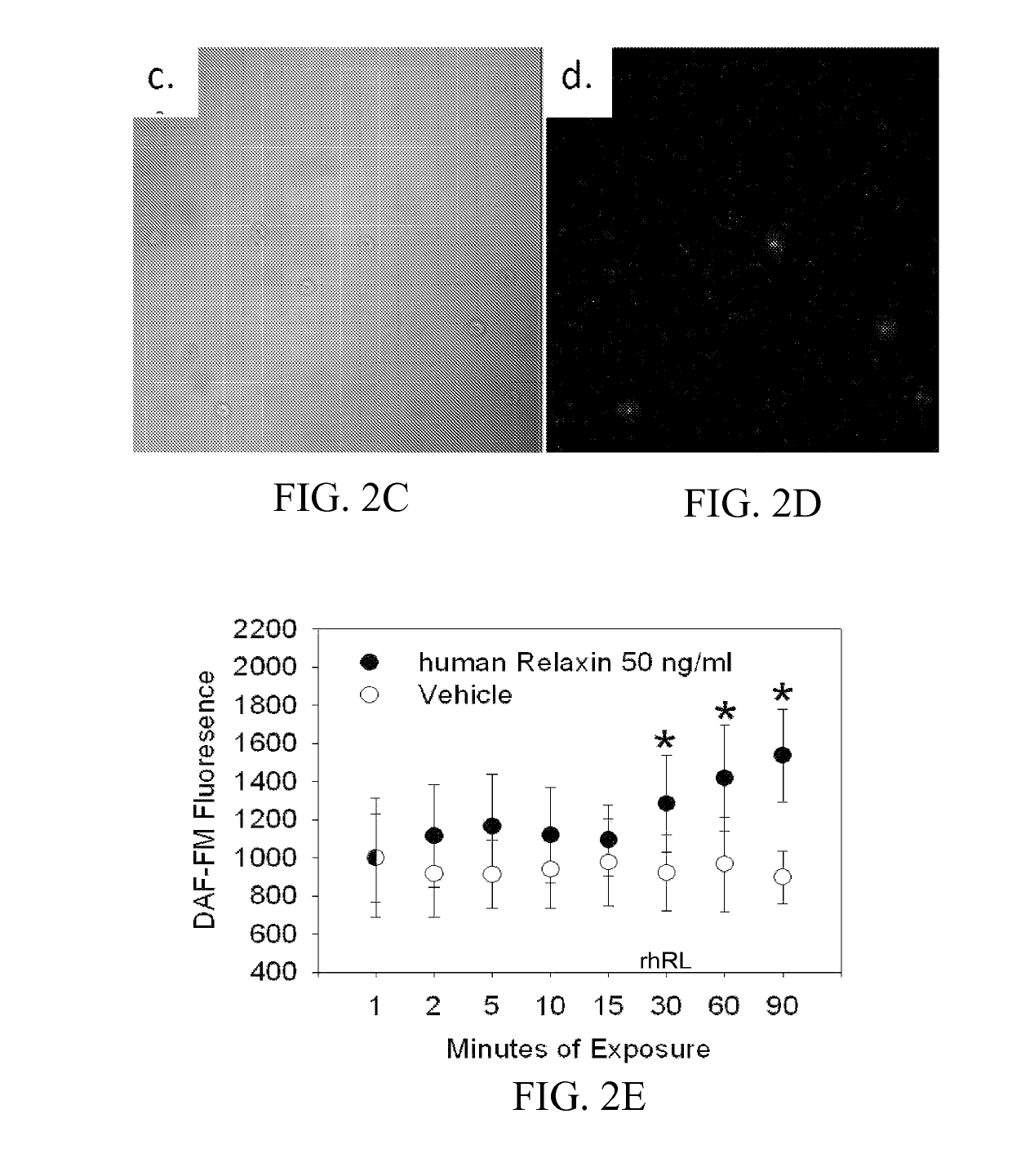
Materials and methods for modulating activity of bone marrow derived cells
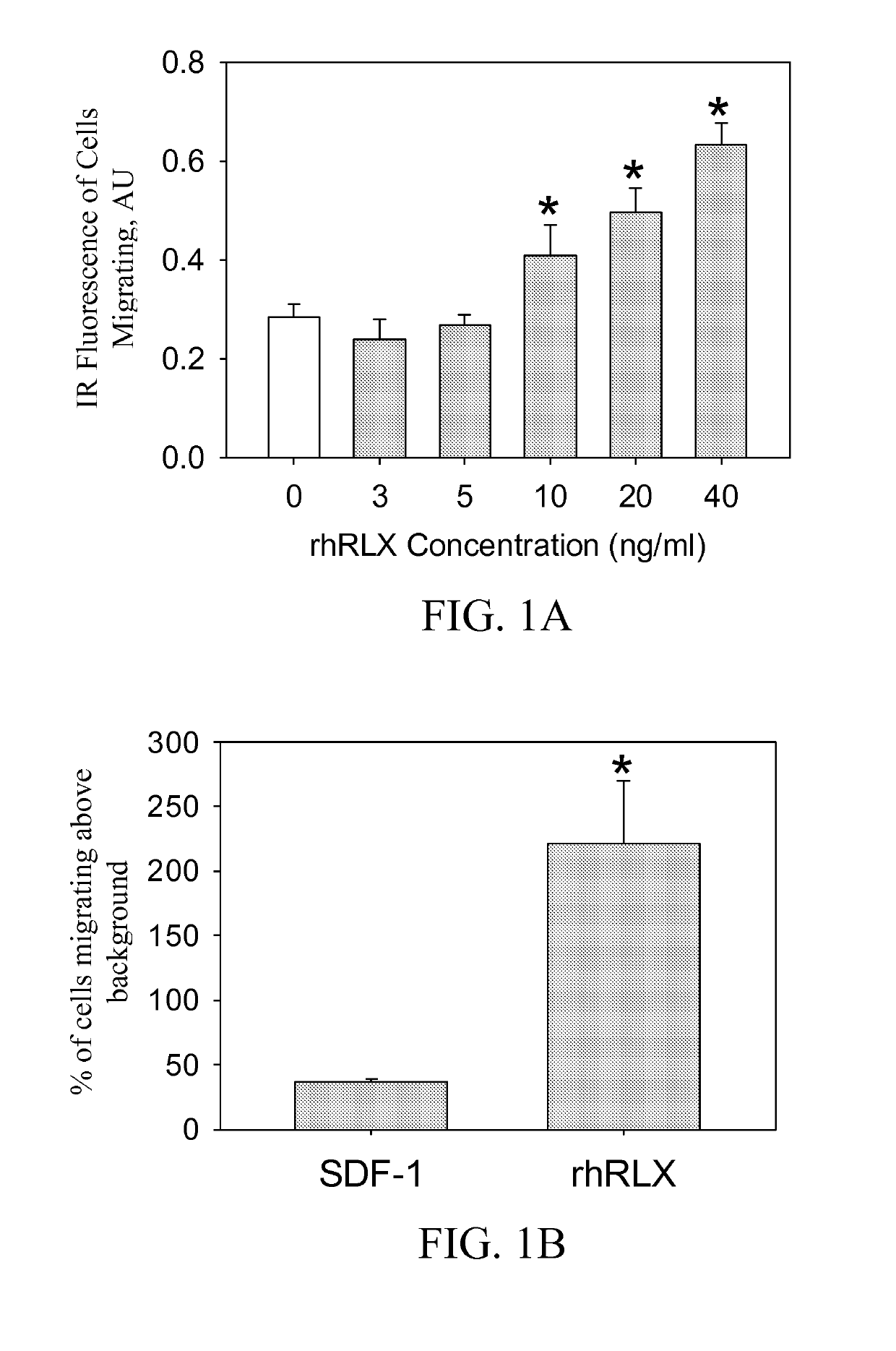
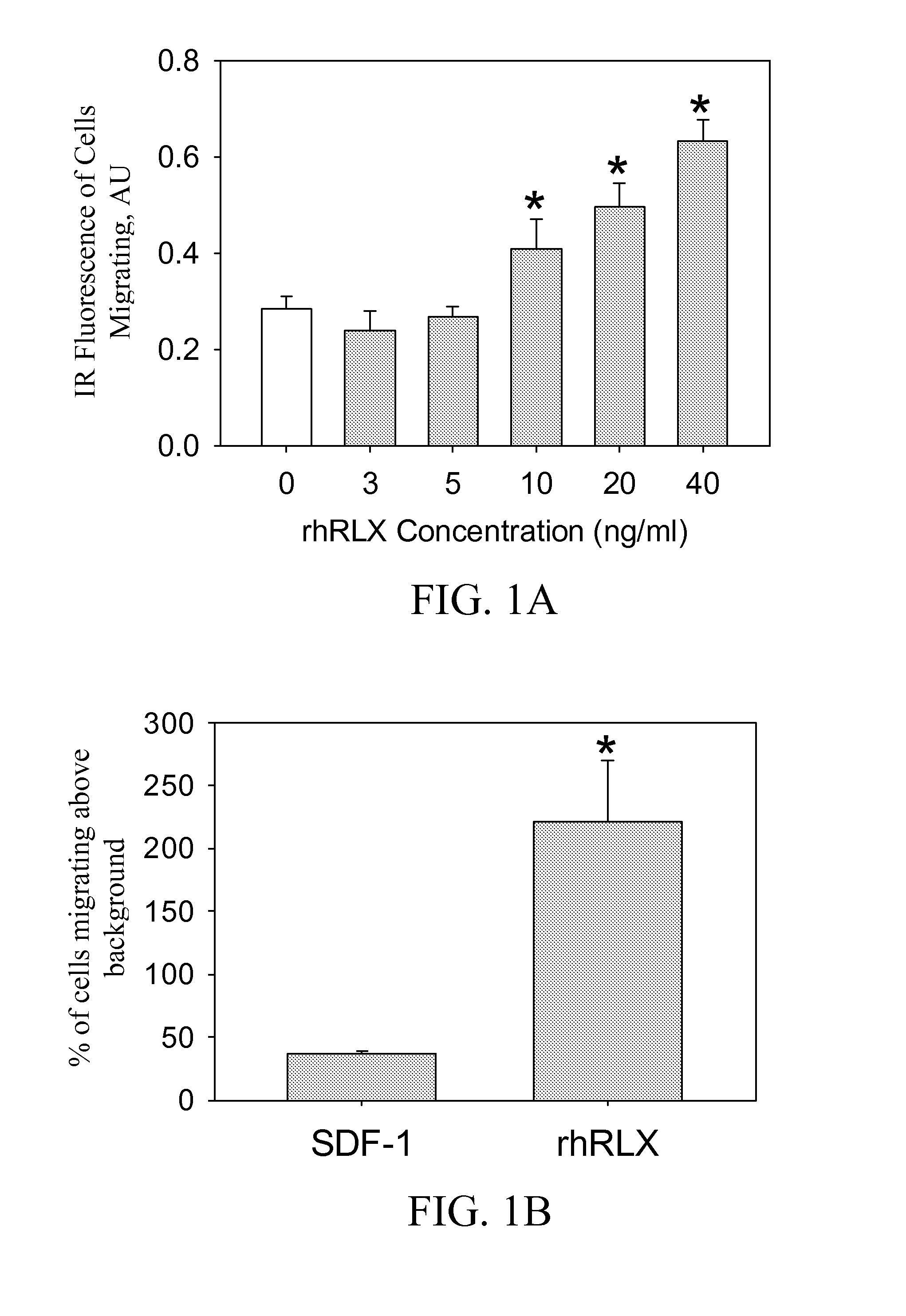
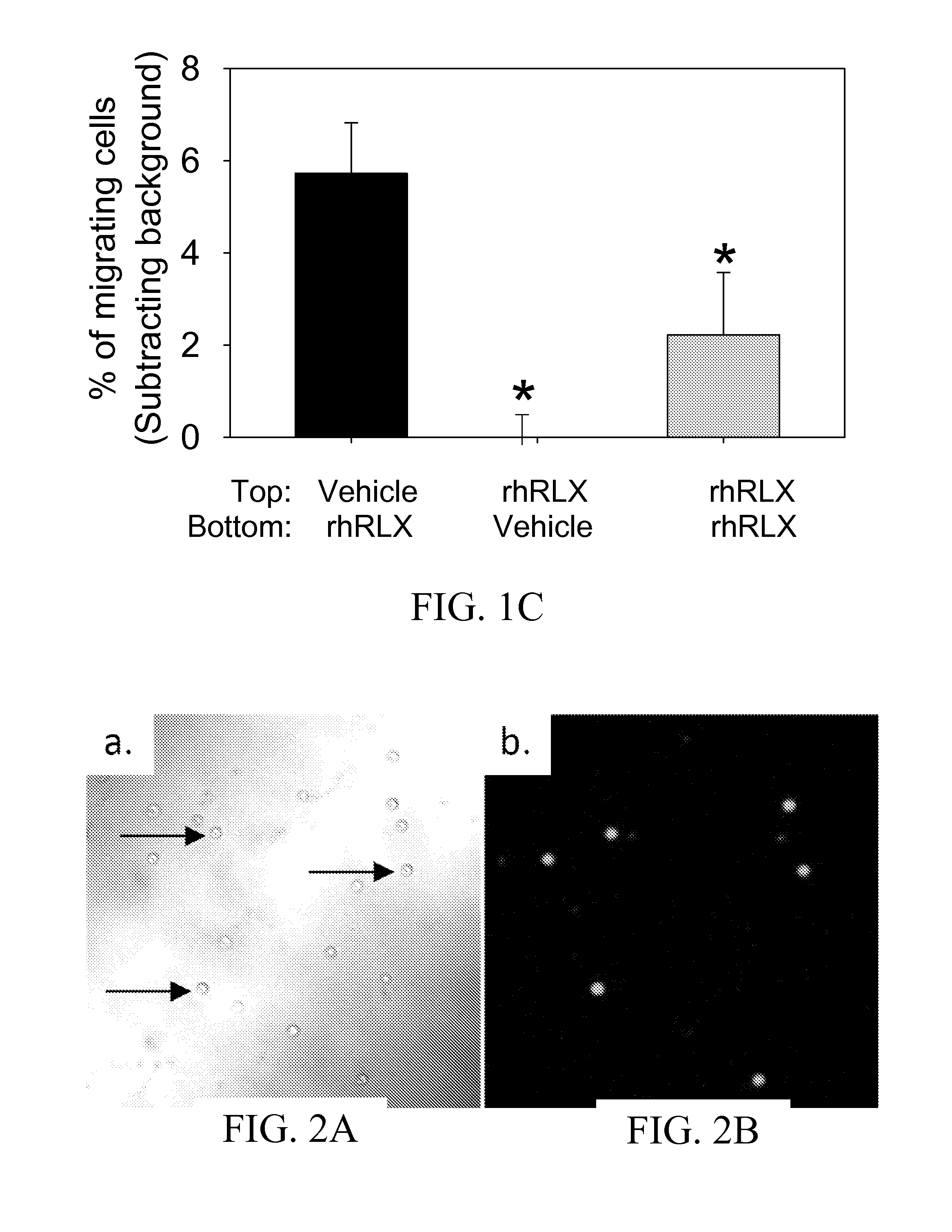
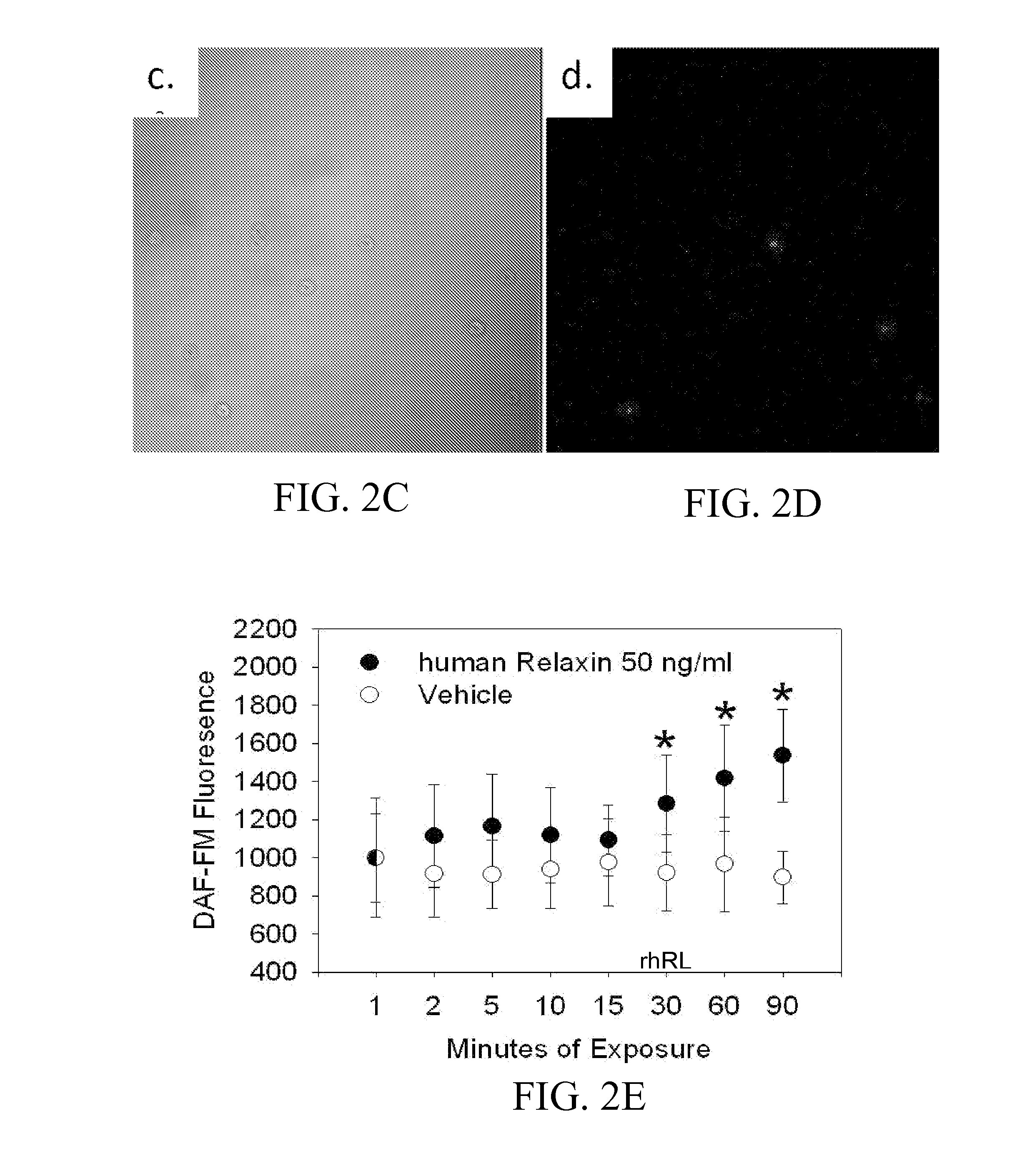
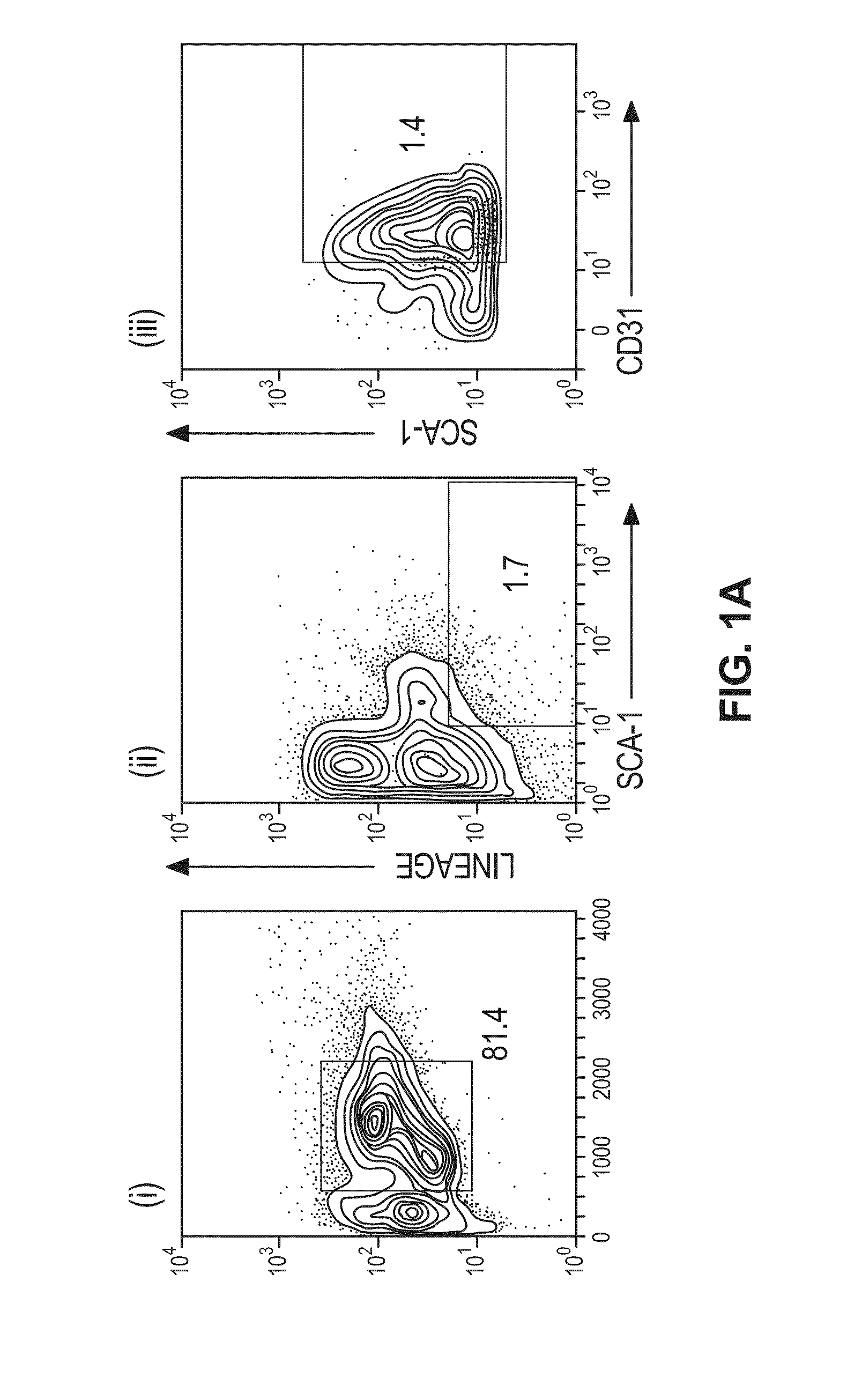
ActiveUS10493131B2Positively impactIncrease blood flowBiocidePeptide/protein ingredientsRelaxinBone Marrow-Derived Cell
The subject invention provides methods for recruitment of bone marrow-derived cells, including bone marrow-derived endothelial cells (BMDEC), and increasing their function by administration of relaxin. The methods of the invention can be used in, for example, treating conditions amenable to treatment by recruitment of bone marrow-derived cells, such as BMDEC and bone marrow-derived angio-osteogcnic progenitor cell.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
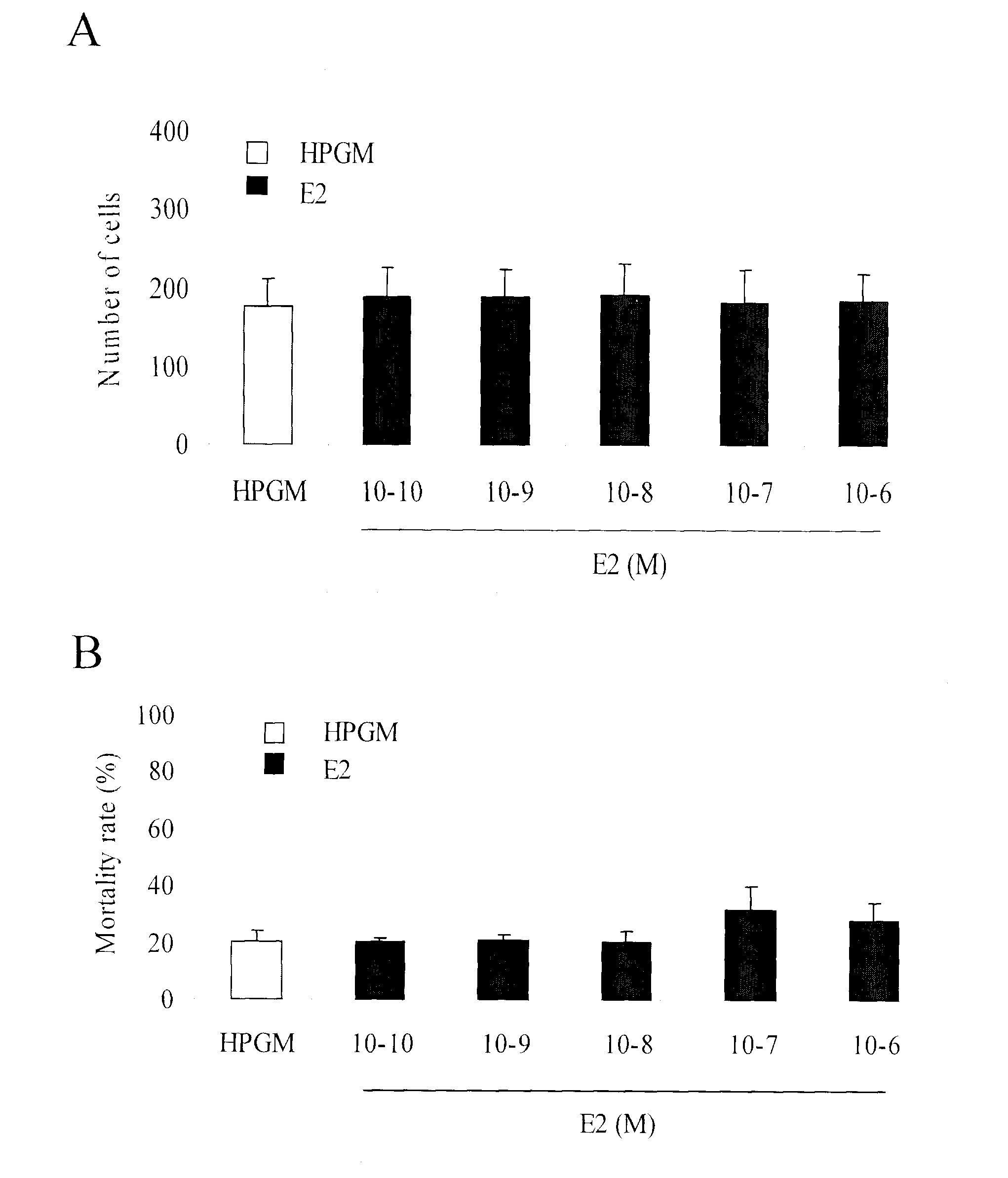
Method of reducing the effects of cytostatic drugs on bone marrow derived cells, and methods of screening
InactiveUS20100247602A1Improve survivalSimple methodOrganic active ingredientsSurgeryAntisense nucleic acidBone Marrow-Derived Cell
A method of using an estrogen receptor agonist and antagonist to reduce a toxic effect of a cytostatic drug on bone marrow derived cells in a biological system. The methods comprise contacting the cells with a therapeutically effective amount of an estrogen receptor agonist or antagonist, and contacting the cells with a cytostatic agent, whereby the toxic effect of the cytostatic drug on bone marrow derived cells is reduced. Agonists disclosed include 17-beta-estradiol. Antagonists disclosed include antisense nucleic acids and selective estrogen receptor modulators (SERMs). Furthermore, uses and medicaments comprising estrogen receptor agonists and antagonists are provided, as are screening methods for identifying therapeutic candidates for reducing the effect of cytostatic agents, and methods of using estrogen receptor agonists for increasing the proliferation of CD117+ cells in a biological system.
Owner:ESTRACURE
Detection of unhealthy bone marrow-derived cell for disease predispositions
InactiveUS20120309019A1Microbiological testing/measurementBiological material analysisInjury mouthFibrosis
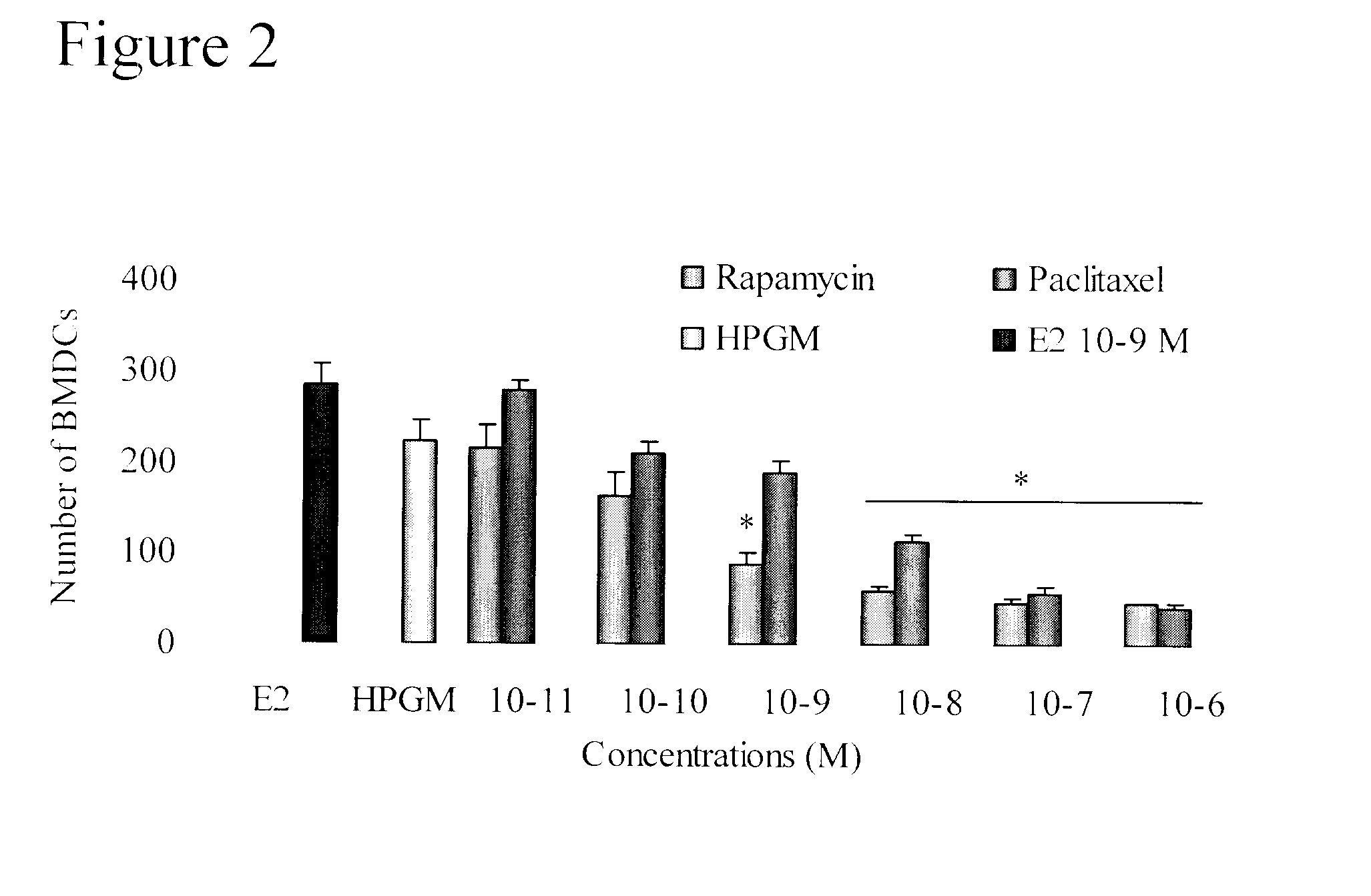
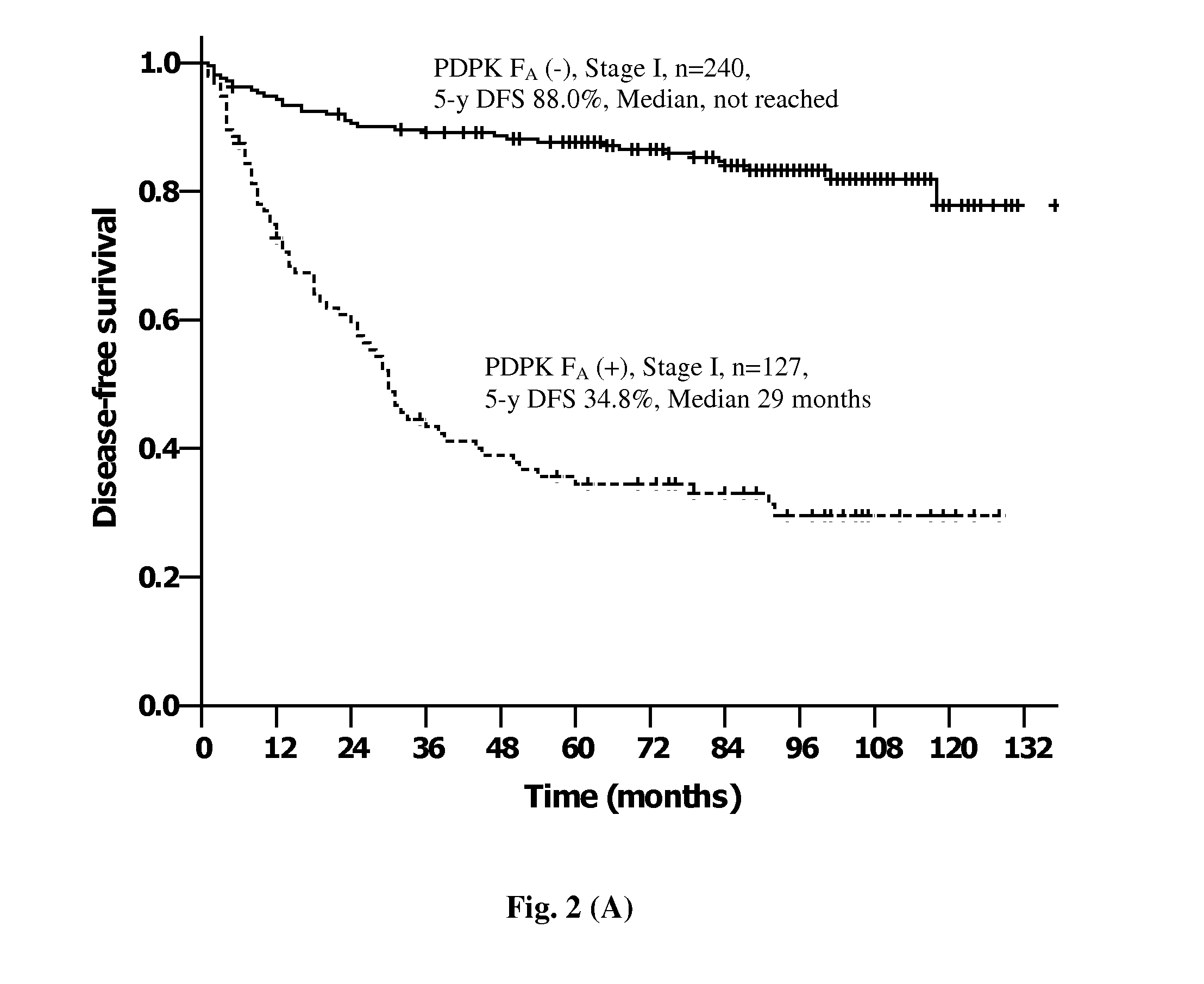
Provided herein is a method for detecting the presence of a unique subset of unhealthy bone marrow-derived cell (BMDC) by co-staining the cytoplasmic and / or nuclear expression of PDPK FA / GSK-3α, and specific BMDC markers as the diagnostic indicator for disease predispositions from very early-stage aberrant wound healing, chronic inflammations, immune disorder towards end-stage fibrotic disease and neoplasia in cancer-free human subjects.
Owner:NATIONAL TSING HUA UNIVERSITY
Materials and methods for modulating activity of bone marrow derived cells
ActiveUS20140242074A1Positively impactIncrease blood flowPeptide/protein ingredientsRelaxinsRelaxinBone Marrow-Derived Cell
The subject invention provides methods for recruitment of bone marrow-derived cells, including bone marrow-derived endothelial cells (BMDEC), and increasing their function by administration of relaxin. The methods of the invention can be used in, for example, treating 5 conditions amenable to treatment by recruitment of bone marrow-derived cells, such as BMDEC and bone marrow-derived angio-osteogcnic progenitor cell.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
CD34+ cells and methods of use
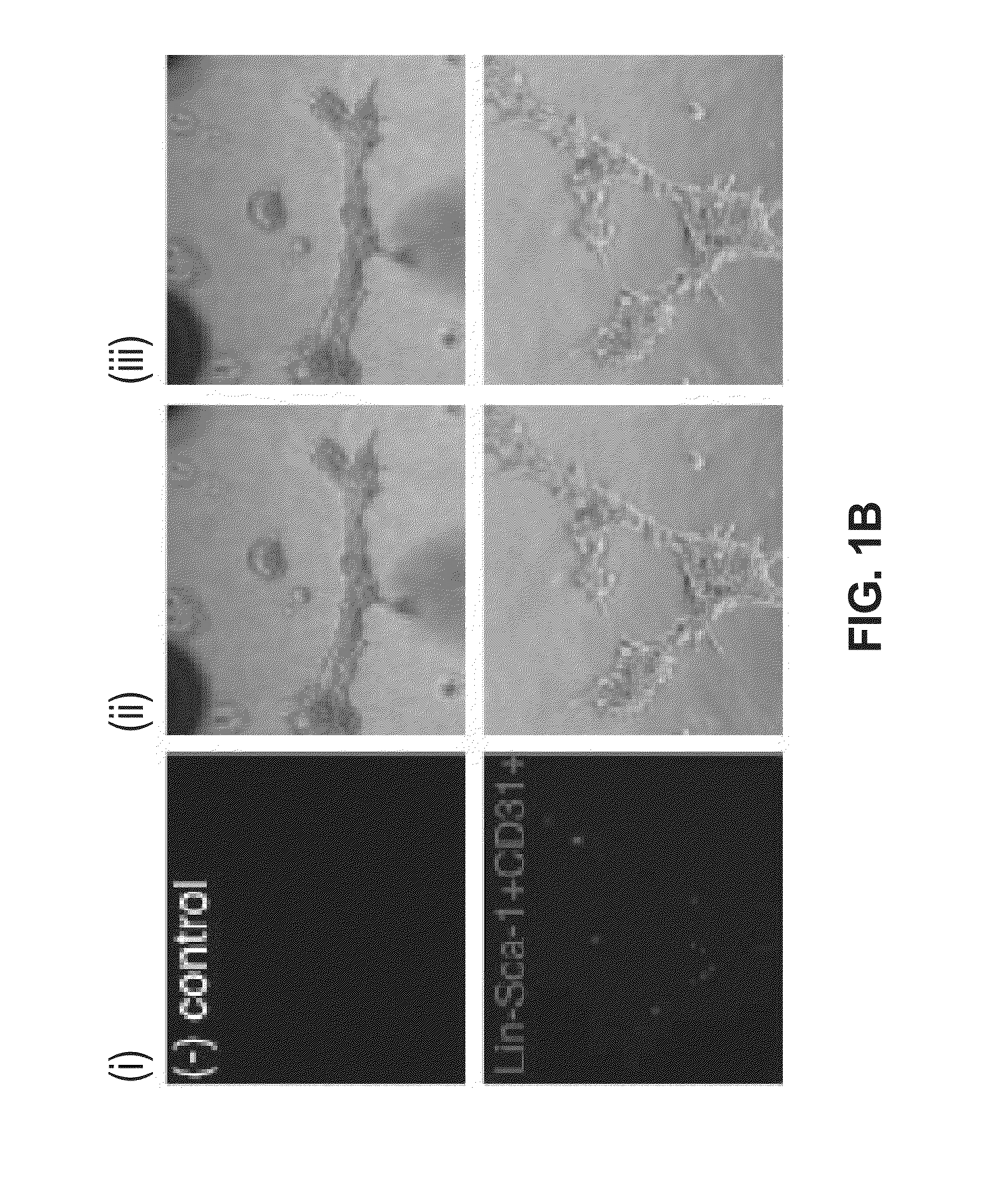
InactiveUS20140134139A1Improve functionalityReduce functionBiocideUnknown materialsCardiac muscleBone Marrow-Derived Cell
Disclosed herein is a reprogrammed endothelial progenitor cell, said cell comprises a bone marrow-derived cell expressing the CD34+ marker and at least one cardiomyocyte-specific gene, as well as methods for preparing and using the same for cardiac regenerative medicine.
Owner:NORTHWESTERN UNIV
Preparation method of medical cartilage support material
ActiveCN103301508BSame mechanical strengthImprove efficiencyProsthesisPorosityBone Marrow-Derived Cell
The invention provides a preparation method of a medical cartilage support material. The preparation method comprises the following operation steps of: pretreatment, separation and primary washing of a cartilage tissue, inactivation of virus, decellularization, sodium chloride treatment, forming, and packaging and sterilization. The bone marrow-derived cell component and DNA (Deoxyribonucleic Acid) component of an animal are completely removed from the medical cartilage support material prepared by the method, meanwhile a natural ECM (Extracellular Cell Matrix) component and a three-dimensional are completely remained, and no endotoxin, organic solvent and toxic solvent are left; the medical cartilage support material has a porosity suitable for cell ingrowth and cartilage tissue regeneration, and also has ideal mechanical properties.
Owner:BEIJING BIOSIS HEALING BIOLOGICAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com