Pharmaceutical for promoting functional regeneration of damaged tissue
A tissue regeneration and cell technology, applied in tissue culture, antipyretics, animal cells, etc., can solve the problems of poor prognosis of life, insufficient effect of immune-gene therapy, etc., and achieve the effect of promoting functional tissue regeneration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
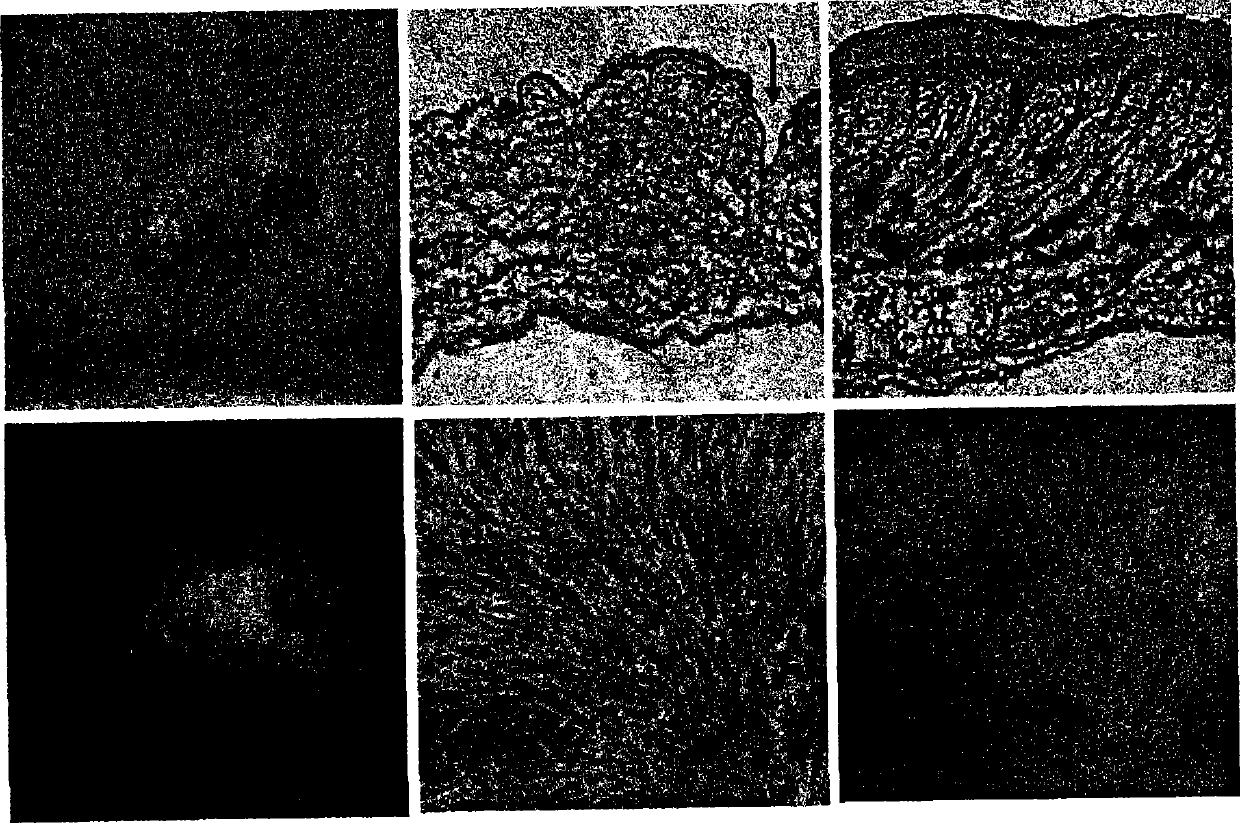
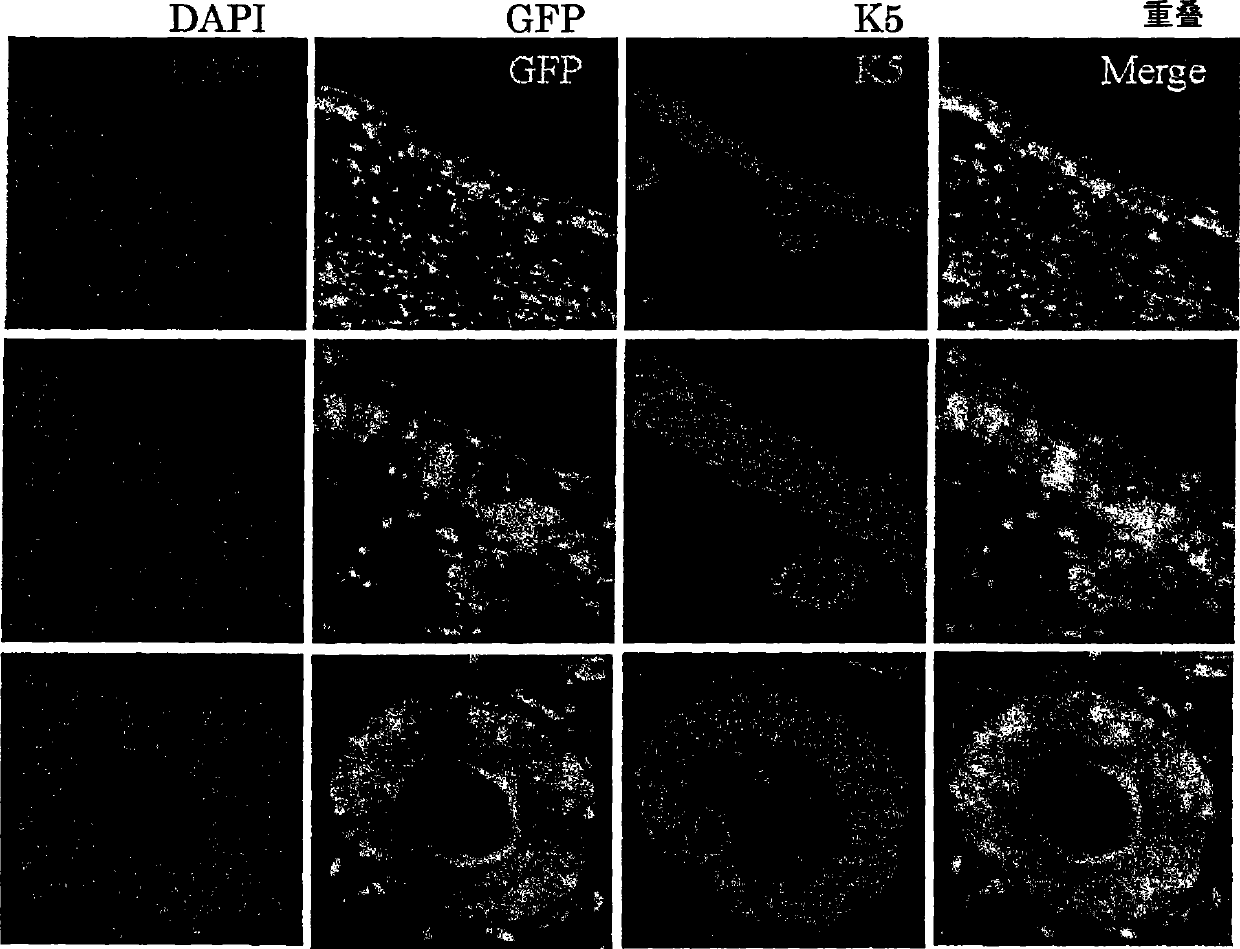
[0259] Objective: To evaluate the role of bone marrow-derived cells in functional regeneration of skin tissue transplanted in living organisms
[0260] Method: For the above purpose, research was carried out by the following method.
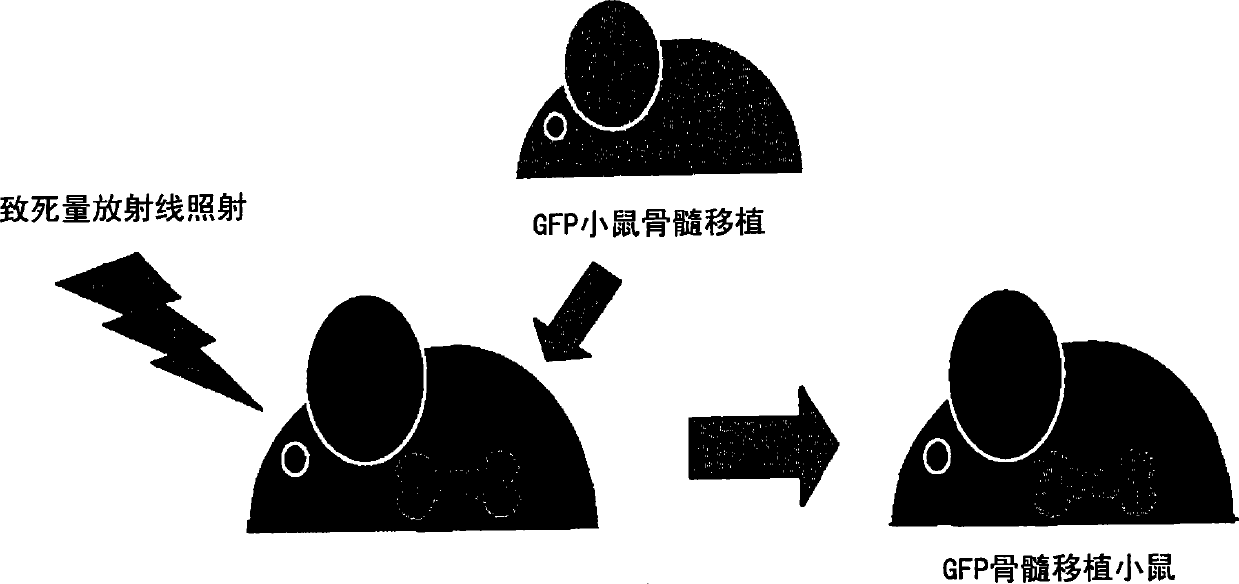
[0261] 1) The degree of contribution of bone marrow-derived cells to the functional regeneration of the grafted skin sheet was investigated using a living skin graft system to GFP bone marrow transplanted mice. Specifically, C57BL / 6 male mice (6-8 weeks old) were irradiated with lethal radiation (10 Gy), and immediately thereafter transplanted GFP (green fluorescent protein) transgenic mouse-derived bone marrow cells (5×10 6 Each / 0.1ml physiological phosphate buffer solution pH7.4)( figure 1 ).
[0262] 2) Waiting for the engraftment of transplanted bone marrow cells (6 weeks), newborn mouse skin (female) was transplanted on the back skin of the obtained GFP bone marrow transplanted mouse.
[0263] 3) Waiting for the implantation of the transp...
Embodiment 2
[0269] Objective: Identification of bone marrow mesenchymal stem cell-inducing factors present in skin tissue extracts
[0270] Method: In order to identify the bone marrow mesenchymal stem cell mobilization factor expected to be released from the resected skin in a state of hypoperfusion, the following method was used for the study.
[0271] 1) To obtain mouse bone marrow-derived mesenchymal stem cells, bone marrow cells of C57BL / 6 mice were collected from the femur or lower leg bone, and D-MEM (manufactured by Nacalai Co., Ltd.) containing 10% fetal bovine serum was used as a cell culture medium. Inoculated in a cell culture dish and cultured at 37° C. and a carbon dioxide concentration of 5%. When the area occupied by the cells grew to 70 to 100% of the bottom area of the culture dish, the cells were detached from the culture dish using 0.25% trypsin 1 mM EDTA (manufactured by Nacalai), and then subcultured under the same conditions. The subculture operation was repeated...
Embodiment 3
[0283] Objective: To identify the HMGB1 family in skin extracts and study the induction activity of bone marrow mesenchymal stem cells
[0284] Method: The presence or absence of the HMGB protein family contained in the skin extract of newborn mice was confirmed by Western blot. As a sample, 10 μl of the skin extract obtained in [Example 2] was used for electrophoresis by SDS-PAGE, and the protein separated in the gel was transferred to a PVDF membrane using a blotting apparatus (ATTO). After incubating at room temperature for 1 hour with PBS (S-T-PBS) containing 3% skimmed milk and 0.1% Tween 20, the rabbit anti-mouse HMGB1 antibody, rabbit anti-mouse HMGB2 antibody, rabbit anti-mouse HMGB2 antibody, and rabbit Anti-mouse HMGB3 antibodies were reacted at 4°C for 16 hours. After the reaction, wash the same PVDF membrane with S-T-PBS for 5 minutes and 5 times, and then use peroxidase-labeled goat anti-rabbit IgG antibody (GE Healthcare) diluted to 2000 times with S-T-PBS, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com