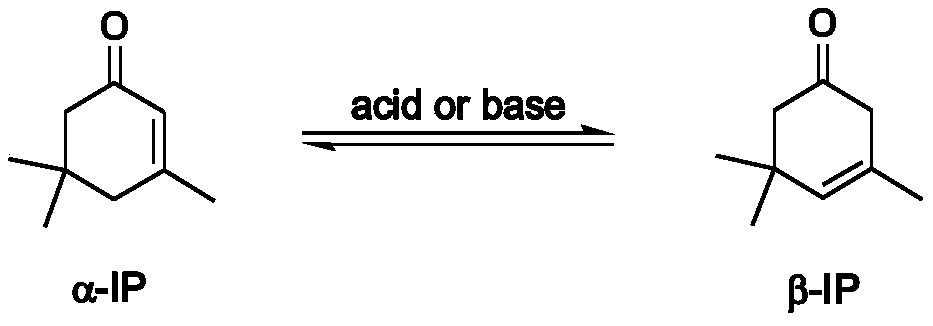
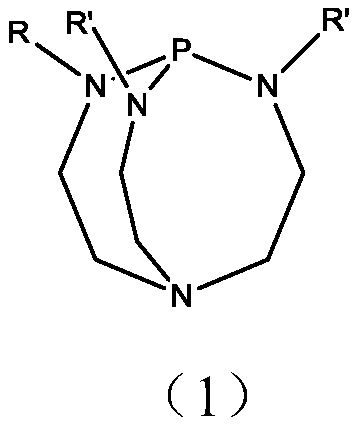
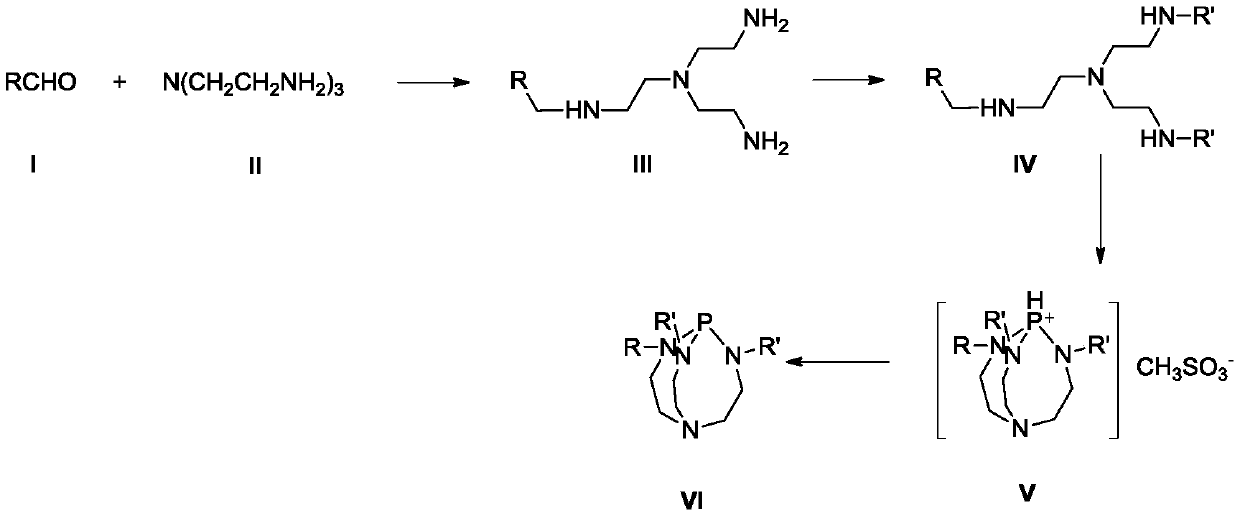
A method for preparing 3,5,5-trimethyl-3-cyclohexen-1-one
A cyclohexene and trimethyl technology, which is applied in the field of preparation of 3,5,5-trimethyl-3-cyclohexene-1-one, can solve the problem of excessive catalyst dosage, low space-time yield, Strong corrosion of equipment, etc., to achieve high isomeric selectivity, reduce the generation of by-products, and reduce the effects of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Add the α-IP raw material containing 0.05wt% pre-nitrogen phosphatrane catalyst IL-A to the tower reactor of the tower reactor, and the temperature of the tower reactor is 210 ° C, the number of theoretical plates is 30, and the reflux ratio is 3:1. Reactive distillation was carried out under the condition of 0.9 Bar absolute pressure in the reactor, α-IP isomerization reaction occurred, the reaction selectivity was 99.7%, and the crude product β-IP was collected at the top of the tower (the gas phase purity was 70wt%). The crude product β-IP is 1.5kPa in absolute pressure, the number of theoretical plates is 30, and the reflux ratio is 3:1, further vacuum distillation to obtain the product β-IP with a purity of 99.5wt%, and the temperature at the top of the column is 100 ℃.
Embodiment 2-10 and Embodiment 11
[0052] Embodiment 2-10 and embodiment 11 (comparative example)
[0053] On the basis of Example 1, change the catalyst type and consumption, the number of theoretical plates of the reactive distillation column reactor, the temperature of the tower bottom, pressure, reflux ratio, residence time, the results are shown in Table 1.
[0054] Table 1
[0055]
[0056]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com