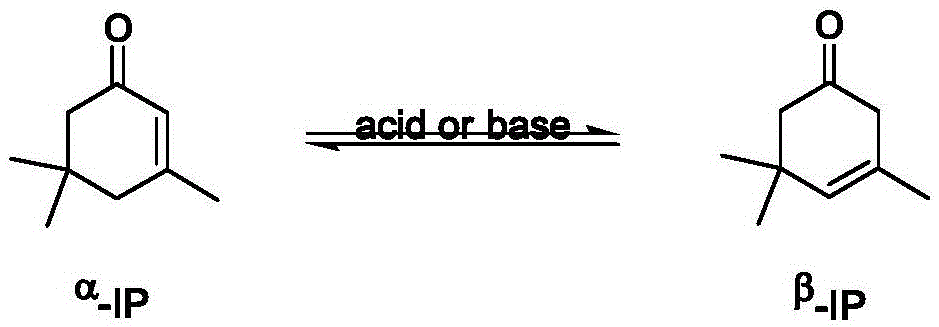A green preparation process of 3,5,5-trimethyl-3-cyclohexen-1-one
A preparation process, the technology of cyclohexene, which is applied in the field of preparation process of alkaline ionic liquid to catalyze the synthesis of β-IP, can solve the problems that the catalyst cannot be recycled, the space-time yield is not high, and the environmental pollution is large, so as to achieve high isomerization selectivity , solve the separation problem and reduce the effect of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation method of alkaline ionic liquid used in the present invention
[0034] The anion potassium salt (K + B — , Purchased from Bailingwei Technology), added to ionic liquid bromide (A + Br — , Purchased from Shanghai Chengjie Chemical Co., Ltd.) in methylene chloride solution, stirred vigorously at room temperature for 10 hours, and then the precipitate potassium bromide (KBr) was filtered off. The filtrate was subjected to rotary evaporation to remove the solvent, and then washed with ether for 3 times, and finally dried at 90°C for 10 hours to obtain the corresponding ionic liquid (A + B — ).
Embodiment 2
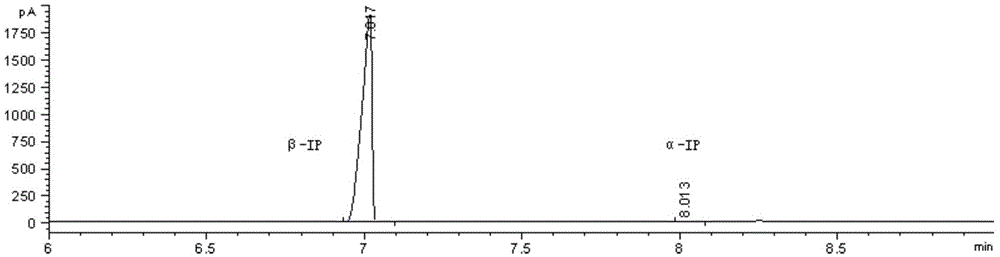
[0036] The α-IP raw material containing 0.05wt% alkaline ionic liquid catalyst IL-A is added to the tower reactor of the tower, and the rectification reaction is carried out under the conditions of 210°C and 0.9 Bar, and the α-IP isomerization reaction occurs and stays The reaction time is 24 hours, the reaction selectivity is 99.7%, and the crude product β-IP (purity: 94 wt%) is collected at the top of the tower. The crude product β-IP is subjected to a pressure of 1.5KPa, 30 plates, and a reflux ratio of 3:1, and further vacuum distillation to obtain a product β-IP with a purity of 99.5% by weight. The gas analysis spectrum is shown in Figure 1. .
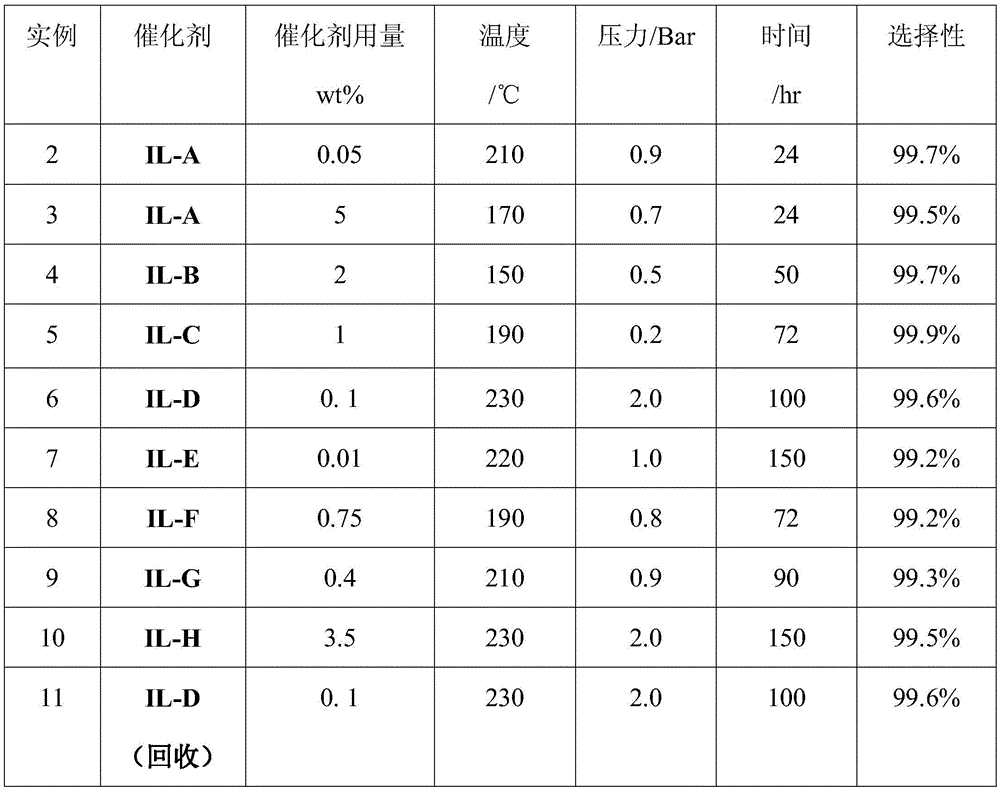
Embodiment 3-10
[0038] On the basis of Example 2, the type and amount of catalyst, temperature, pressure, and reaction time were changed. The results are shown in Table 1.
[0039] Among them, the structural formulas of the catalysts IL-F, IL-G and IL-H are respectively
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com