A kind of preparation method of β-isophorone
A technology of isophorone and trimethyl, which is applied in the preparation of organic compounds, carbon-based compounds, chemical instruments and methods, etc., can solve the problems of high content of β-IP-2, affecting the quality of downstream VE, and low production efficiency. Advanced problems, to achieve the effect of stable catalytic system, low catalyst consumption and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
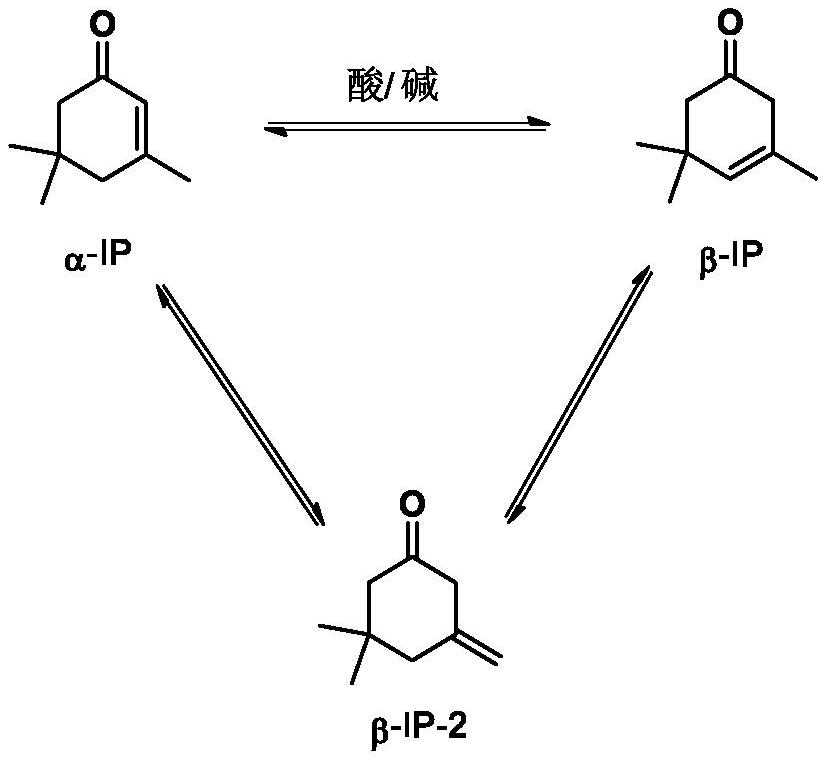
[0029] Add the α-IP raw material containing 0.05wt% n-hexanoic acid+cyclohexylamine (mol ratio is 1:1) catalyst to the tower reactor tower reactor, at the tower reactor temperature 210 ℃, the number of theoretical plates is 30, and the reflux ratio is 30:1, reactive distillation was carried out under the condition of absolute pressure of 0.9Bar in the tower reactor, α-IP isomerization reaction occurred, and the reaction selectivity was 99.7%, and β-IP (gas phase purity was 97.0wt%) was collected from the top of the tower. ).
Embodiment 2-10 and Embodiment 11
[0030] Example 2-10 and Example 11 (comparative example)
[0031] On the basis of Example 1, the catalyst type and amount, the theoretical plate number of the reactive distillation column reactor, the temperature of the tower kettle, the pressure, the reflux ratio, and the residence time were changed. The results are shown in Table 1.
Embodiment 11
[0032] Example 11 (comparative example)
[0033] catalyst The preparation method:
[0034] Add potassium carbonate to in dichloromethane solution (the molar ratio of the two is 1:2), vigorously stirred at room temperature for 10 hours, and then the precipitated potassium bromide (KBr) was filtered off. The filtrate was evaporated to remove the solvent, washed three times with ether, and finally dried at 90 °C for 10 h to obtain the corresponding ionic liquid.
[0035] Table 1
[0036]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com