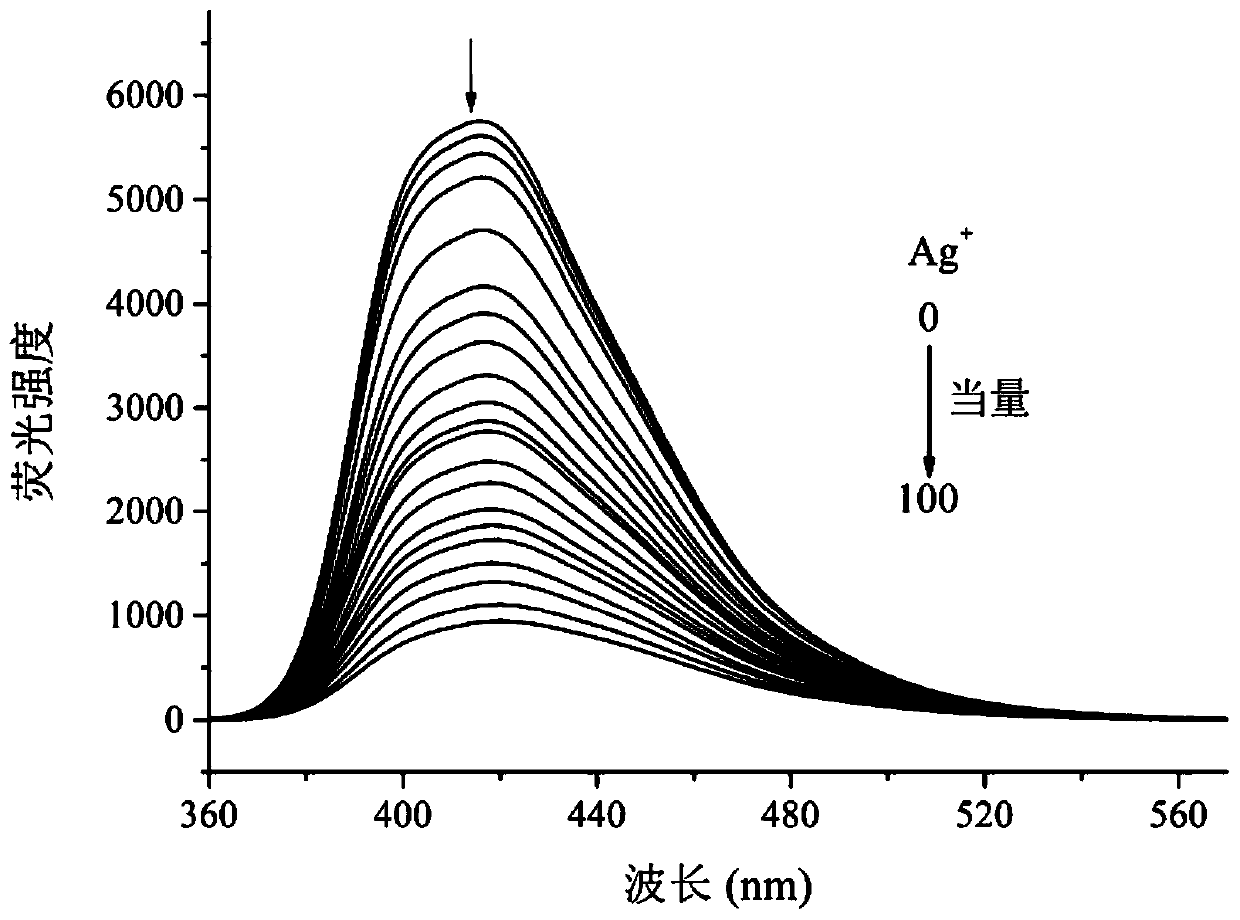
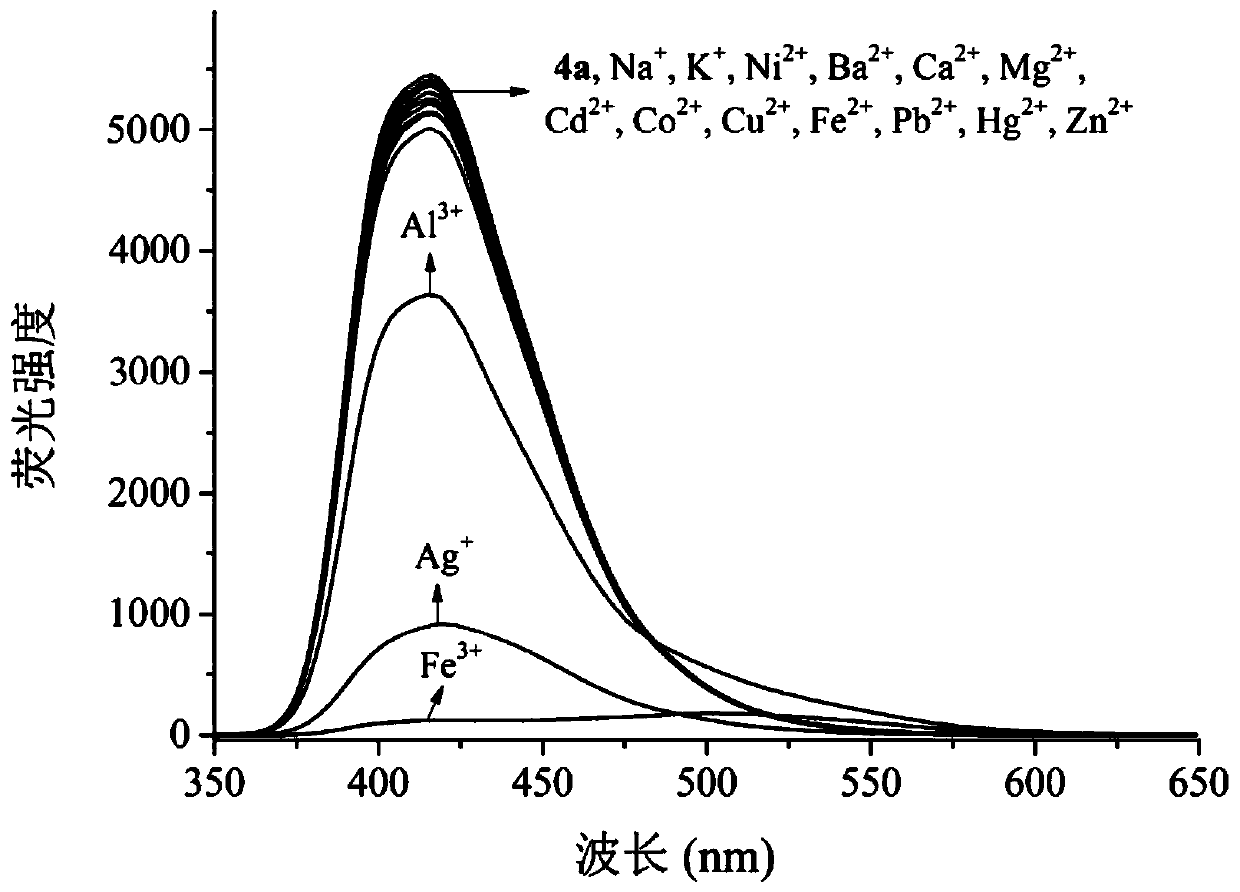
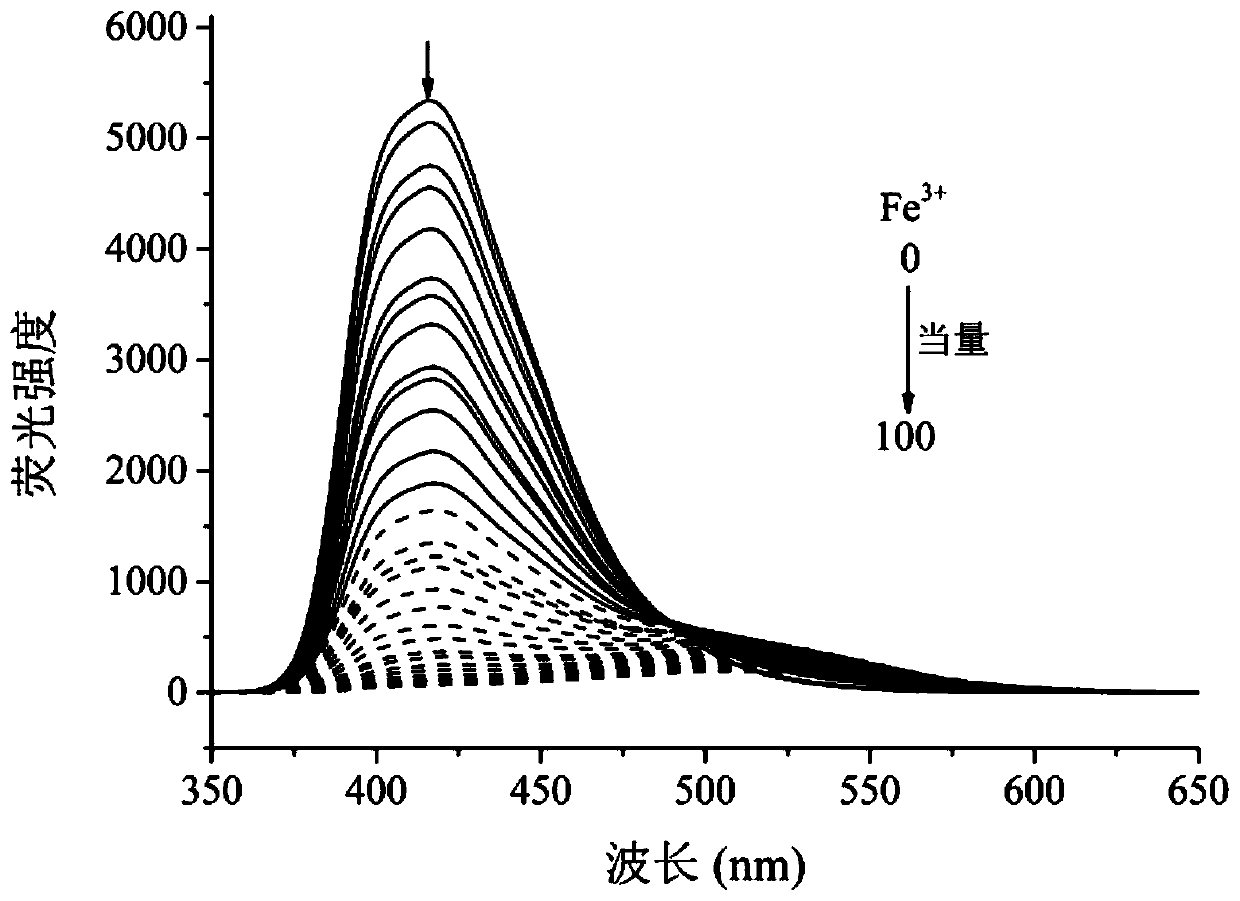
A kind of multifunctional bis(benzimidazole) naphthalene fluorescent chemical sensor and its application
A chemical sensor, benzimidazole technology, applied in the field of fluorescence detection, achieves the effect of simple approach, simple process and rapid synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] (1) With 1.393 grams of 1-bromodecane and 1.249 grams of 2,6-two (5,6-dimethylbenzimidazole) naphthalene (that is, in the structural formula of two (benzimidazole) naphthalene compounds, benzimidazole group Located at the 2,6-position of the naphthalene ring, and R 1 =CH 3 ) as raw material, according to the amount of substance n (1-bromodecane):n (2,6-two (5,6-dimethylbenzimidazole) naphthalene) = 2.1:1 mixed uniformly, at a temperature of 80 Under the condition of ℃, 0.480 gram of NaOH solid as catalyst and 30 milliliters of acetonitrile as solvent, N-alkylation substitution reaction was carried out for 4 hours;
[0057] (2) After the reaction, dissolve and transfer with ethyl acetate, take the organic phase after washing several times, dry over anhydrous magnesium sulfate, spin off the solvent, and the crude product is separated by column chromatography to obtain a white solid multifunctional bis(benzene) and imidazole) naphthalene fluorescent chemical sensor 1.694...
Embodiment 2
[0065] (1) With 1.570 grams of 1-bromododecane and 1.249 grams of 2,6-two (5,6-dimethylbenzimidazole) naphthalene (that is, in the structural formula of two (benzimidazole) naphthalene compounds, benzimidazole The group is located at the 2,6-position of the naphthalene ring, and R 1 =CH 3 ) as raw material, by the amount of substance n (1-bromododecane): n (2,6-two (5,6-dimethylbenzimidazole) naphthalene) = 2.1:1 mixed uniformly, at a temperature of 80 DEG C, 0.480 grams of NaOH solid as a catalyst and 30 milliliters of acetonitrile as a solvent, carry out N-alkylation substitution reaction for 4 hours;
[0066] (2) After the reaction, dissolve and transfer with ethyl acetate, take the organic phase after washing several times, dry over anhydrous magnesium sulfate, spin off the solvent, and the crude product is separated by column chromatography to obtain a white solid multifunctional bis(benzene) and imidazole) naphthalene fluorescent chemical sensor 1.785 grams.
[0067] ...
Embodiment 3
[0074] (1) With 1.747 grams of 1-bromotetradecane and 1.249 grams of 2,6-two (5,6-dimethylbenzimidazole) naphthalene (that is, in the structural formula of the aforementioned two (benzimidazole) naphthalene compounds, benzo The imidazolyl is located at the 2,6-position of the naphthalene ring, and R 1 =CH 3 ) as raw material, by the amount of substance n (1-bromotetradecane): n (2,6-two (5,6-dimethylbenzimidazole) naphthalene) = 2.1:1 mixed uniformly, at a temperature of 80 DEG C, 0.480 grams of NaOH solid as a catalyst and 30 milliliters of acetonitrile as a solvent, carry out N-alkylation substitution reaction for 4 hours;
[0075] (2) After the reaction, dissolve and transfer with ethyl acetate, take the organic phase after washing several times, dry over anhydrous magnesium sulfate, spin off the solvent, and the crude product is separated by column chromatography to obtain a white solid multifunctional bis(benzene) and imidazole) naphthalene fluorescent chemical sensor 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com