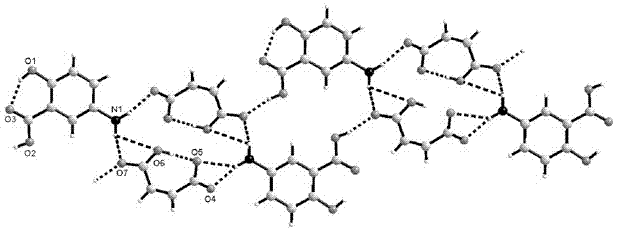
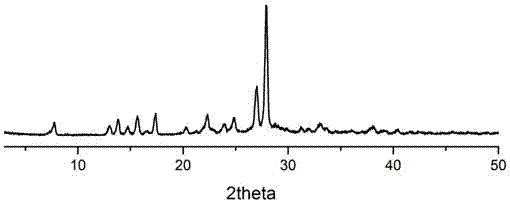
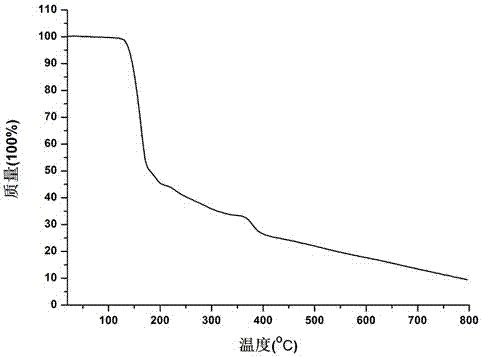
5-amino salicylic acid pharmaceutical co-crystal and preparation method thereof
A technology of aminosalicylic acid and aminobenzoic acid, which is applied in the field of drug co-crystals, can solve problems such as difficulty in obtaining high-purity crystals, high cost and technical requirements, and products that are easily affected by solvents, so as to extend the market cycle, Effects of improved solubility and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Weigh 30mg of 5-aminosalicylic acid and 68.1mg of maleic acid in an agate mortar, grind them evenly, add 20μL of methanol, grind quickly until the solvent evaporates, then transfer the mixture to a glass test tube, add 2mL of acetone and 2mL of A mixed solvent of methanol, ultrasonicated until completely dissolved, sealed the test tube mouth and pierced a small hole to stand for volatilization for 6 days, crystals began to precipitate at the bottom of the test tube, and 46 mg of light yellow transparent block crystals were obtained, which were 5-aminosalicylic acid and maleic acid drugs Eutectic with a yield of 89%.
Embodiment 2
[0038] Weigh 30 mg of 5-aminosalicylic acid and 30.2 mg of 2,6-dihydroxybenzoic acid and place them in an agate mortar, grind them evenly, add 20 μL of methanol, grind quickly until the solvent evaporates, and then transfer the mixture to a glass test tube, Add a mixed solvent of 2mL acetone and 2mL methanol, sonicate until it is completely dissolved, seal the test tube mouth and pierce a small hole and let it stand for volatilization for 3 days. Crystals start to precipitate at the bottom of the test tube, and 50 mg of light yellow transparent block crystals are obtained as 5-aminosalicylic acid The drug was co-crystallized with 2,6-dihydroxybenzoic acid with a yield of 83%.
Embodiment 3
[0040] Weigh 30 mg of 5-aminosalicylic acid and 18.4 mg of 4-aminopyridine and place them in an agate mortar, grind them evenly, add 20 μL of methanol, grind quickly until the solvent evaporates, then transfer the mixture to a glass test tube, add 2 mL of acetone and 2mL of methanol mixed solvent, sonicated until completely dissolved, sealed the test tube mouth and pierced a small hole to stand for volatilization for 5 days, crystals began to precipitate at the bottom of the test tube, and 27 mg of light yellow transparent blocky crystals were obtained, which were 5-aminosalicylic acid and 4-aminosalicylic acid Pyridine drug co-crystal with a yield of 56%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com