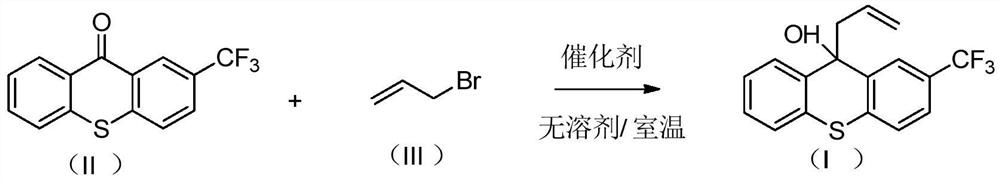
A kind of preparation method of 2-trifluoromethyl-9-allyl-9-thioxanthol
A technology of trifluoromethyl and thioxanthol, applied in the field of preparation of 2-trifluoromethyl-9-allyl-9-thioxanthol, which can solve environmental pollution, long reaction time, increased production costs, etc. problems, achieve the effects of reducing production costs, avoiding the use of reaction solvents, and reducing emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add tin powder (17.81g, 0.15mol) to a 500mL reaction bottle, and add 2-trifluoromethyl-9-thioxanthone (28.03g, 0.10mol) dropwise while stirring at room temperature (25°C). ) and allyl bromide (18.15g, 0.15mol).
[0026] After the dropwise addition, keep stirring at room temperature (25°C) for 1-2 hours. After the reaction, slowly add the reaction mixture dropwise into a 1L reaction flask containing 280.0g of saturated ammonium chloride solution and 225g of ethyl acetate. After dripping, keep stirring for 30 minutes, let stand to separate the liquid, collect the organic layer, remove the solvent from the organic layer under reduced pressure, and obtain 30.9 g of light brown oily liquid 2-trifluoromethyl-9-allyl-9-thioxanthol , yield 95.8%, purity (HPLC area normalization method) 90.0%.
Embodiment 2
[0028] Add tin powder (15.43g, 0.13mol) to a 500mL reaction bottle, and add 2-trifluoromethyl-9-thioxanthone (28.03g, 0.10mol) dropwise while stirring at room temperature (25°C). ) and allyl bromide (18.15g, 0.15mol).
[0029] After the dropwise addition, keep stirring at room temperature (25°C) for 1-2 hours. After the reaction, slowly add the reaction mixture dropwise into a 1L reaction flask containing 250.0g of saturated ammonium chloride solution and 200g of ethyl acetate. After dripping, keep stirring for 30 minutes, let stand to separate the liquid, collect the organic layer, remove the solvent from the organic layer under reduced pressure, and obtain 30.7 g of light brown oily liquid 2-trifluoromethyl-9-allyl-9-thioxanthol , yield 95.2%, purity (HPLC area normalization method) 98.6%.
Embodiment 3
[0031] Add tin powder (17.81g, 0.15mol) to a 500mL reaction bottle, and add 2-trifluoromethyl-9-thioxanthone (28.03g, 0.10mol) dropwise while stirring at room temperature (25°C). ) and allyl bromide (12.10g, 0.10mol) mixture.
[0032] After the dropwise addition, keep stirring at room temperature (25°C) for 1-2 hours. After the reaction, slowly add the reaction mixture dropwise into a 1L reaction flask containing 250.0g of saturated ammonium chloride solution and 200g of ethyl acetate. After dropping, keep stirring for 30 minutes, let stand to separate the liquid, collect the organic layer, remove the solvent from the organic layer under reduced pressure, and obtain 29.3 g of light brown oily liquid 2-trifluoromethyl-9-allyl-9-thioxanthol , yield 90.8%, purity (HPLC area normalization method) 96.5%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap