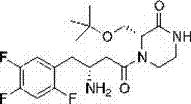
Application of asymmetric conjugate addition reaction in synthesis of Imigliptin intermediate compound
A technology of conjugated addition and intermediates, which is applied in the field of synthesis of epagliptin intermediates, can solve the problems of low product yield, high cost, poor quality, etc., and achieve simple synthesis methods, good product quality, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0028] Preparation of compound (3)
[0029] Add 20 L of reaction solvent anhydrous dichloromethane and 2.6 kg (10 mol) of compound (2) and 1.2 kg (11 mol) of phenylhydroxylamine to the 50L reactor at room temperature, then add catalyst 84 g (0.1 mol) After the addition, the temperature was raised to reflux, TLC followed the reaction, and the reaction was completed after 22 hours. The reaction solution was added to 20L 5% ammonium chloride aqueous solution, separated, and the organic phase was concentrated under reduced pressure to obtain the crude product of compound (3). The crude product was washed with toluene 3.5 kg (9.49 mol) of the refined product were obtained by recrystallization, and the yield was 94.9%. Purity by HPLC: 97.4% (ee 98.7%).
[0030] 1 H NMR (400 MHz, DMSO- d 6 ) δ 7.24 – 7.05 (m, 2H), 6.97 – 6.83 (m, 1H),6.83 – 6.73 (m, 3H), 6.61 (m, 1H), 3.51 (m, 1H), 3.39 – 3.08 (m, 3H ), 2.82(m, 1H), 2.72 – 2.47 (m, 2H), 1.61 (s, 1H), 1.35 (t, J = 6.6 Hz, 3H). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com