Method, reagents, and kits for detecting minimal residual disease
A reagent and antibody technology, applied in the field of cancer diagnosis, can solve problems such as hindering the detection of low-frequency malignant cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
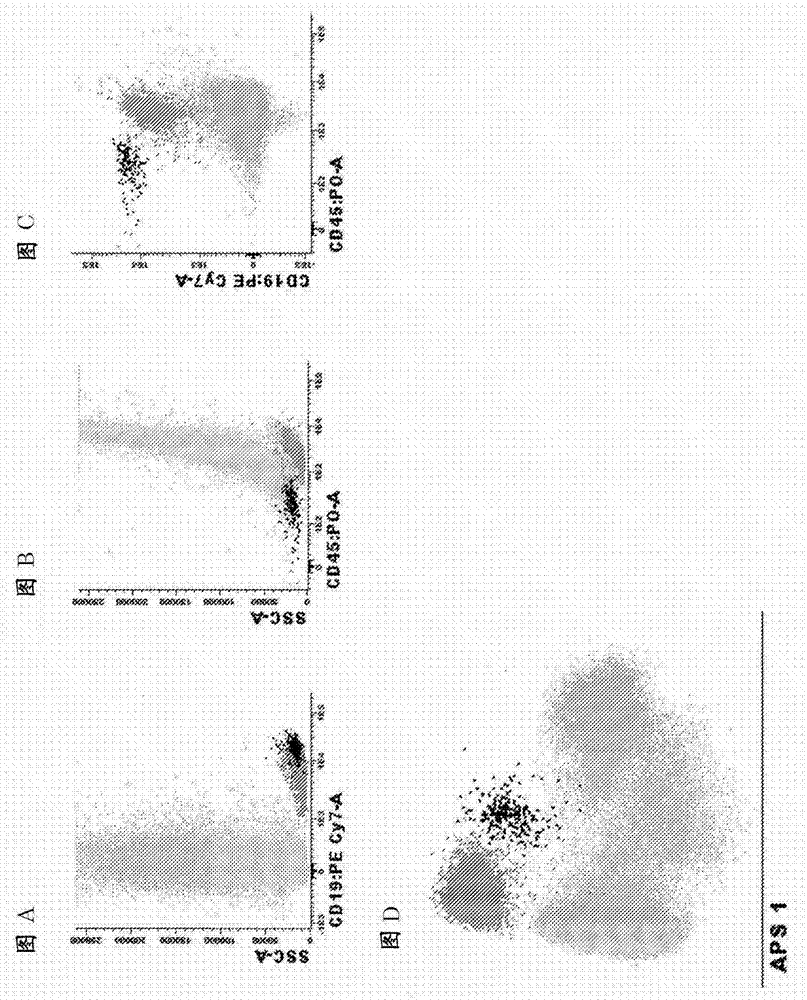
[0096] Example 1. Antibody panels and diagnostic methods for MRD detection in BCP-ALL patients
[0097] Markers for the identification of total B cells and B cell precursors in the bone marrow
[0098] List of relevant identification markers : CD19, CD45
[0099] how to use them : Pre-gating using the CD19 marker is necessary to identify pure B cell populations. To focus on normal B-cell precursors (BCPs), CD45-negative or weakly positive CD45-positive mature B cells can be distinguished from BCPs. In the case of CD19-directed therapy, CD22 can be used instead of CD19. These markers can also be used in combination with side light scatter (SSC) or forward light scatter (FSC) or both FSC and SSC to identify peripheral blood or bone marrow or other types of samples (e.g. bone marrow, tissue biopsy, spinal fluid) B cells in. Of note, additional markers (such as CD10, CD20, CD38, and CD34) (see below) that differentiate normal BCP cells from BCP-ALL cells (see below) can ...
Embodiment 2
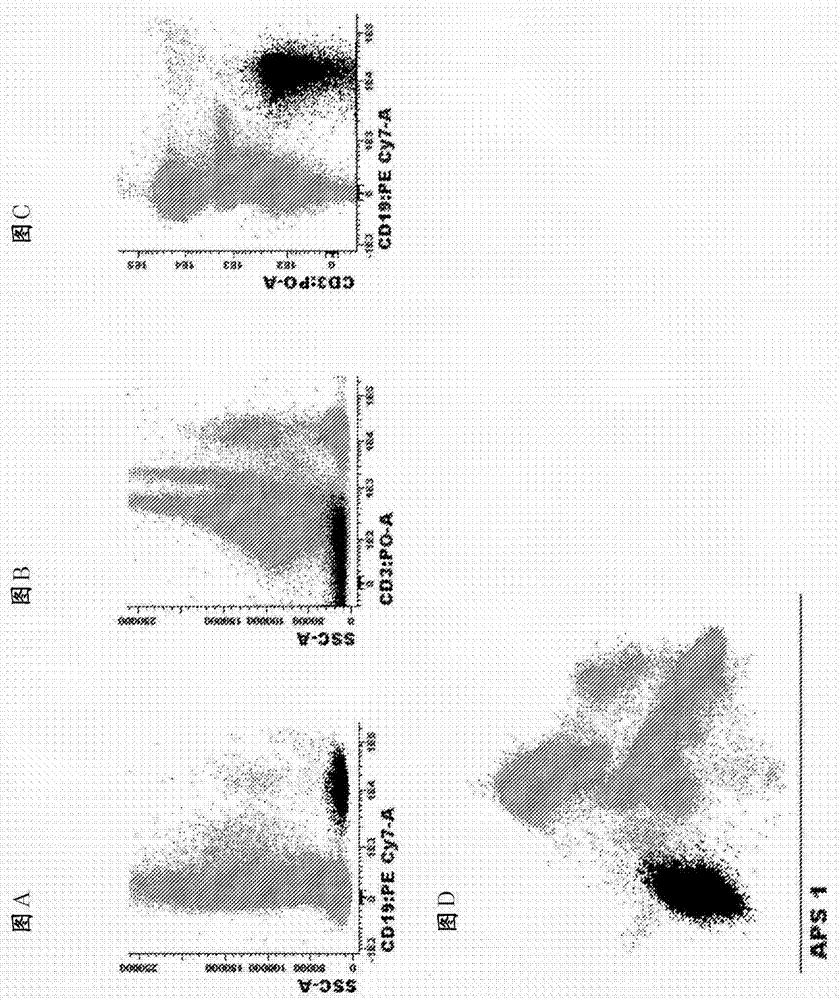
[0114] Example 2. Antibody panels and diagnostic methods for MRD detection in CLL patients
[0115] Markers used to identify total B cells in peripheral blood and bone marrow:
[0116] list of identification markers : CD19, CD3 (markers excluded)
[0117] how to use them : Pre-gating with this marker combination is necessary to identify pure B cell populations and remove T cell / B cell doublets. These markers can also be used in combination with side light scatter (SSC) or forward light scatter (FSC) or both FSC and SSC to identify of B cells. For finer gating to better enrich for CLL cells, both CD5 and CD27 can be used. Markers used to differentiate normal B cells from CLL cells:
[0118] List of markers and most common phenotypic aberrations :
[0119] CD27 : Positive on CLL cells and a small fraction of normal B cells
[0120] CD5 : Positive on CLL cells and a small fraction of normal B cells
[0121] CD79b : Underexpressed on CLL cells compared to norm...
Embodiment 3
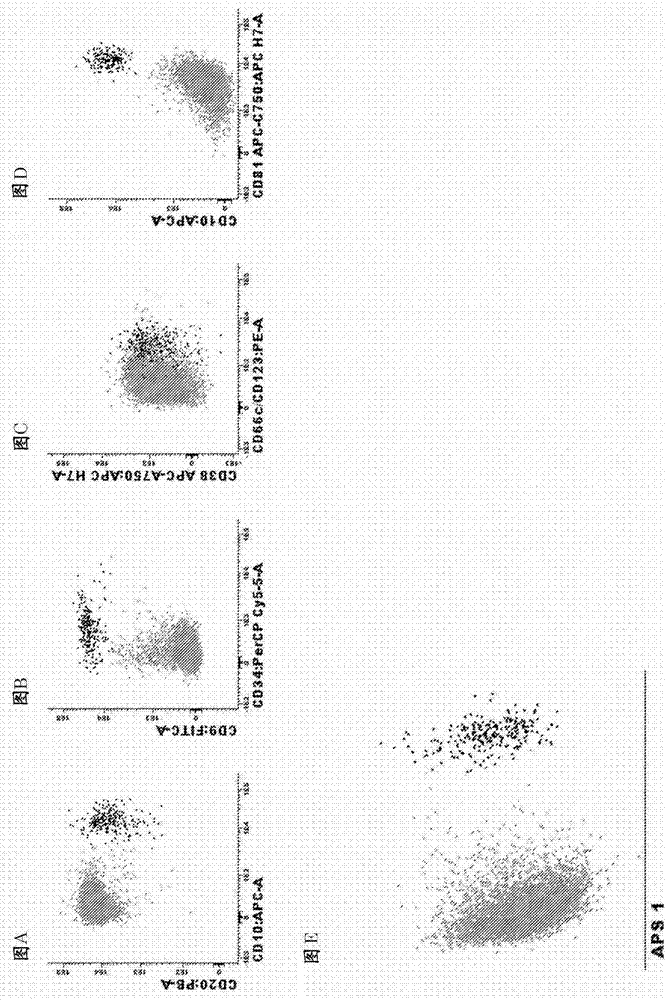
[0129] Example 3. Antibody panels and diagnostic methods for MRD detection in MM / PCD patients
[0130] Markers used to identify total plasma cells in bone marrow:
[0131] list of identification markers : CD38, CD138 and CD229
[0132] How to use them:Any combination of the three markers works at any fluorochrome position; any combination of two of the three markers may also be used or in some cases (not all) even one of the three markers alone may be used. The combination is preferably in the following order: 1) CD138 / CD38 / CD229; 2) CD138 / CD38, 3) CD138 / CD229; 4) CD38 / CD229; 5) CD138; 6) CD38); 7) CD229. It should be noted that any of these markers alone and in combination can also be used in combination with side light scatter (SSC) or forward light scatter (FSC) or both FSC and SSC to identify bone marrow or other types of samples (e.g. peripheral blood, Plasma cells in tissue biopsy, spinal fluid).
[0133] Markers to differentiate normal from clonal / malignant pla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com