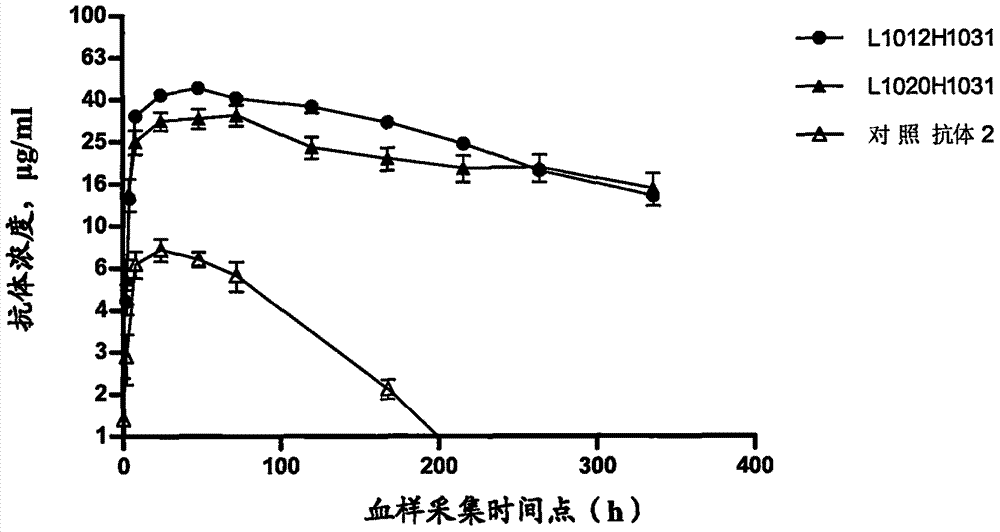
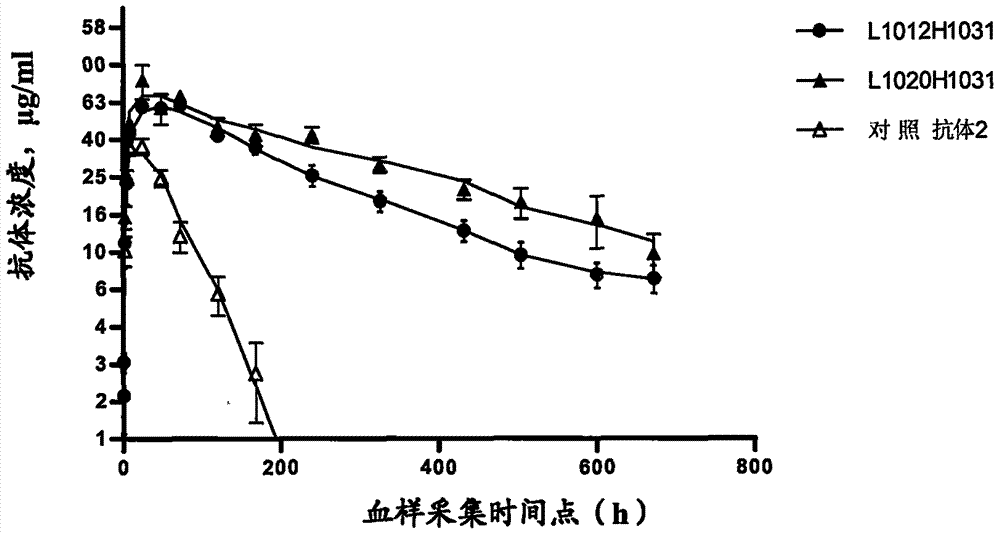
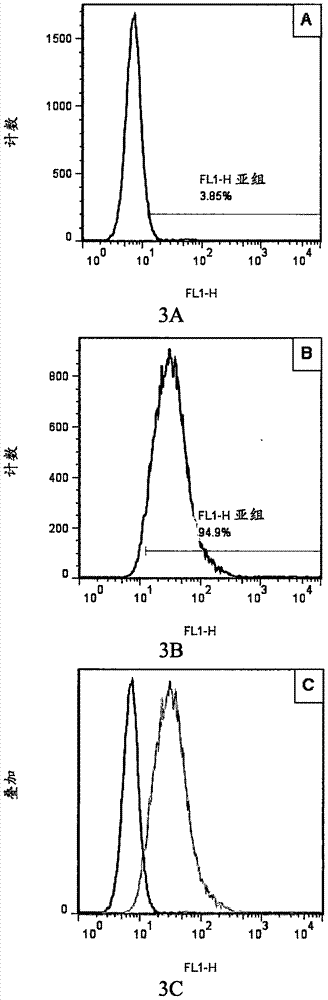
Antibody capable of combining interleukin 4 acceptor
A technology of interleukin and antibody, applied in the direction of fusion cells, antibodies, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0176] Example 1 : Preparation of the antibody of the present invention and non-reducing SDS-PAGE gel electrophoresis to determine the expression level
[0177] The coding sequence of the antibody light chain variable region was inserted into the pFUSE2ss-CLIg-hK vector (Invivogen, product number: pfuse2ss-hclk) using EcoRI and BsiWI restriction sites to form a light chain expression vector; the coding sequence of the heavy chain variable region was used EcoRI and NheI restriction sites were inserted into pFUSEss-CHIg-hG2 vector (Invivogen, product number: pfusess-hchg2) or pFUSEss-CHIg-hG4 vector (Invivogen, product number: pfusess-hchg4) to constitute the heavy chain expression vector.
[0178] Expi293 cells were cultured and transfected according to Invitrogen Expi293 TM Expression SystemKit Manual (Cat. No. A14635). Cell density was adjusted to 2×10 for transfection 6 cells / ml, 0.6 μg light chain expression vector and 0.4 μg heavy chain expression vector were added to...
Embodiment 2
[0189] Example 2 : Detecting that the antibody of the present invention inhibits the proliferation of hIL-4 or hIL-13 on TF-1 cells
[0190] 1. Preparation of reagents
[0191] hIL-4 (Invivogen, product number: rhil-4) solution: dissolve hIL-4 with 100 μl of PBS solution containing 0.1% BSA (Beyotime, product number: ST023) to obtain a concentration of 100 μg / ml, and dissolve the dissolved hIL-4 according to A volume of 5 μl per tube was dispensed into 1.5ml (Nunc) centrifuge tubes and stored in a -20°C refrigerator.
[0192] WST-1 (Beyotime, product number: C0036) solution: Add 5ml of electronic couplant (C0036-2) to WST-1 powder (C0036-1), dissolve completely to form WST-1 solution, and divide according to the volume of 620μl per tube Pack into a 1.5ml centrifuge tube and store in a -20°C refrigerator.
[0193] 2. TF-1 cell culture
[0194] Take out the frozen TF-1 in liquid nitrogen (ATCC: CRL-2003 TM ) cells were shaken in a 37°C water bath to rapidly dissolve them. ...
Embodiment 3
[0215] Example 3 : ELISA detects the binding ability of the antibody of the present invention to sIL-4Rα
[0216] 1. Preparation of reagents
[0217] sIL-4Rα (PEPRO TECH, product number: 200-04R) solution: Take a tube of sIL-4Rα, add 1ml ddH2O, mix upside down to get a 100μg / ml solution, then store in -20℃ refrigerator after aliquoting.
[0218] Sample to be tested: get the Expi293 cell culture supernatant (the culture medium is Expi293Expression Medium, Invitrogen, article number: A1435102; in 8% CO2 incubator, continuous 100rpm suspension culture 4 days) 10 μ l and 2 μl was added to 990 μl and 998 μl of PBS solution respectively to prepare 1:100 and 1:500 diluted antibody samples to be tested.
[0219] Control sample: normal cell culture supernatant (Expi293 cells were not transfected, and the culture medium was Expi293Expression Medium (Invitrogen, article number: A1435102; in 8% CO2 incubator, continuous 100rpm suspension culture for 4 days) was also carried out at 1:10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com