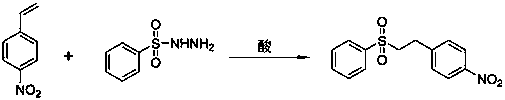
Synthetic method of eletriptan intermediate 4-nitrophenyl-2-(4-benzenesulfonyl hydrazide)ethane
A technology of eletriptan and benzenesulfonyl hydrazide, which is applied in the field of preparation of eletriptan intermediate 4-nitrophenyl-2-ethane, can solve the difficult problem of intermediate N-substituted aldehyde compounds acquisition, high cost of raw materials, high production cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
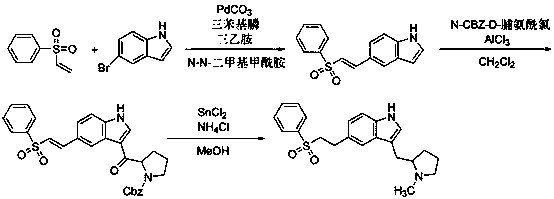
[0014] 1 embodiment one: add benzenesulfonyl hydrazide 0.086g (0.50mmol) in the glass sealing tube of 10mL, p-nitrostyrene 0.298g (2.00mmol), then add dropwise 1mL water, 98% concentrated sulfuric acid 0.0029g (0.03 mmol), the reaction was carried out under magnetic stirring at 80 °C for 5 h. Stop heating, cool naturally, extract with ethyl acetate, rotary evaporate, and then use a silica gel column to separate, the chromatographic liquid is a mixed solvent of n-hexane: ethyl acetate (v / v=30:1), and use n-hexane for recrystallization: A mixed solvent of ethyl acetate (v / v=10:1) was used to obtain 0.132 g of 4-nitrophenyl-2-(4-benzenesulfonylhydrazide)ethane with a yield of 90.7% and a purity of 95.6%.
[0015] 2 Example 2: Add 0.086g (0.50mmol) of benzenesulfonylhydrazide and 0.298g (2.00mmol) of p-nitrostyrene to a 10mL glass sealed tube, then add dropwise 1mL of water, 0.0029g (0.03mmol) of concentrated hydrochloric acid , and reacted with magnetic stirring for 5 h at 80 °C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com