Catalyst for preparing dimethyl carbonate and method for preparing dimethyl carbonate
A technology of dimethyl carbonate and propylene carbonate, which is applied in the field of catalysts for the preparation of dimethyl carbonate, can solve the problems of difficult precise control of the time of propylene glycol stripping, environmental pollution, and reduction of propylene glycol yield, so as to increase the yield of dimethyl carbonate. The overall technical level of the ester industry, the effect of solving key technical problems and reducing the pressure of environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 prepares dimethyl carbonate
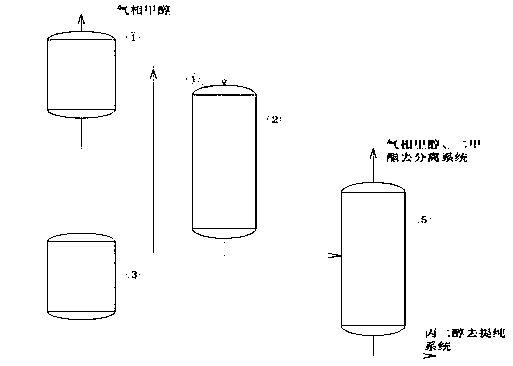
[0033] A device for preparing dimethyl carbonate, comprising a tubular methanol vaporizer (with a heat exchange area of 10㎡), a propylene carbonate preheater (with a heat exchange area of 10㎡), a tubular reactor (with a diameter of 2 meters, 6 meters high, tube diameter 32 mm) and flash tank, such as figure 1 As shown, wherein, the tubular methanol vaporizer is connected to the top of the tubular reactor; the top of the tubular reactor is provided with a mist distributor, and the propylene carbonate preheater is communicated with the tubular reactor through the mist distributor ; The bottom of the tube-and-tube reactor communicates with the flash tank.
[0034] Using the above-mentioned device to prepare dimethyl carbonate: feed methanol into the tubular methanol vaporizer, heat, control the pressure at 0.2Mpa, and the vaporized methanol enters the tubular reactor. Heat the propylene carbonate preheater to raise the tempe...
Embodiment 2
[0037] Embodiment 2 prepares dimethyl carbonate
[0038] Pass methanol to the tubular methanol vaporizer, heat up, control the pressure at 0.2Mpa, and the vaporized methanol enters the tubular reactor. Heat the propylene carbonate preheater to raise the temperature of the propylene carbonate to 110-120°C, and enter the tube-and-tube reactor through the mist distributor. The shell-and-tube reactor is filled with a catalyst, and reacts at a temperature of 115° C. and a pressure of 0.2 MPa. A sample was taken from the bottom of the reactor for analysis, and the content of propylene carbonate was 0.2%. The prepared dimethyl carbonate enters the flash tank from the bottom of the reactor for purification (the gas enters the gas phase methanol and dimethyl ester to remove the separation system, and the liquid phase enters the propylene glycol to remove the purification system). After calculation, the dimethyl carbonate yield 99.7%.
[0039] The mass ratio of methanol and propylene...
Embodiment 3
[0041] Embodiment 3 prepares dimethyl carbonate
[0042] Pass methanol to the tubular methanol vaporizer, heat up, control the pressure at 0.2Mpa, and the vaporized methanol enters the tubular reactor. Heat the propylene carbonate preheater to raise the temperature of the propylene carbonate to 110-120°C, and enter the tube-and-tube reactor through the mist distributor. The shell-and-tube reactor is filled with a catalyst and reacted at a temperature of 110° C. and a pressure of 0.2 MPa. A sample was taken from the bottom of the reactor for analysis, and the content of propylene carbonate was 0.1%. The prepared dimethyl carbonate enters the flash tank from the bottom of the reactor for purification (the gas enters the gas phase methanol and dimethyl ester to remove the separation system, and the liquid phase enters the propylene glycol to remove the purification system). After calculation, the dimethyl carbonate yield 99.8%.
[0043] The mass ratio of methanol and propylene...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com