A kind of preparation method of metal oxide or metal composite oxide
A metal composite and oxide technology, applied in the preparation of oxide/hydroxide, metal/metal oxide/metal hydroxide catalyst, rare earth metal oxide/hydroxide, etc., can solve the problem that the co-precipitation conditions are difficult to control And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
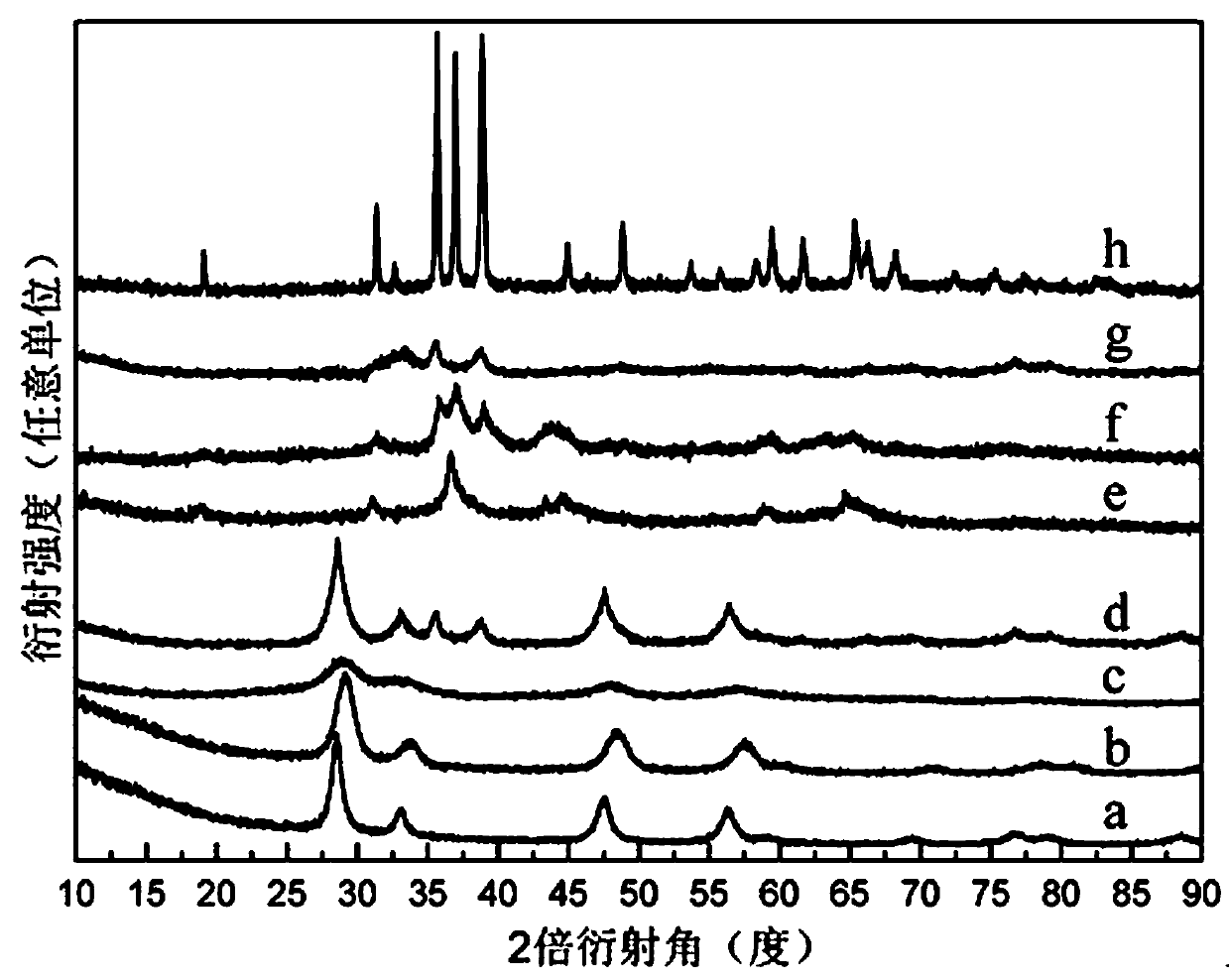
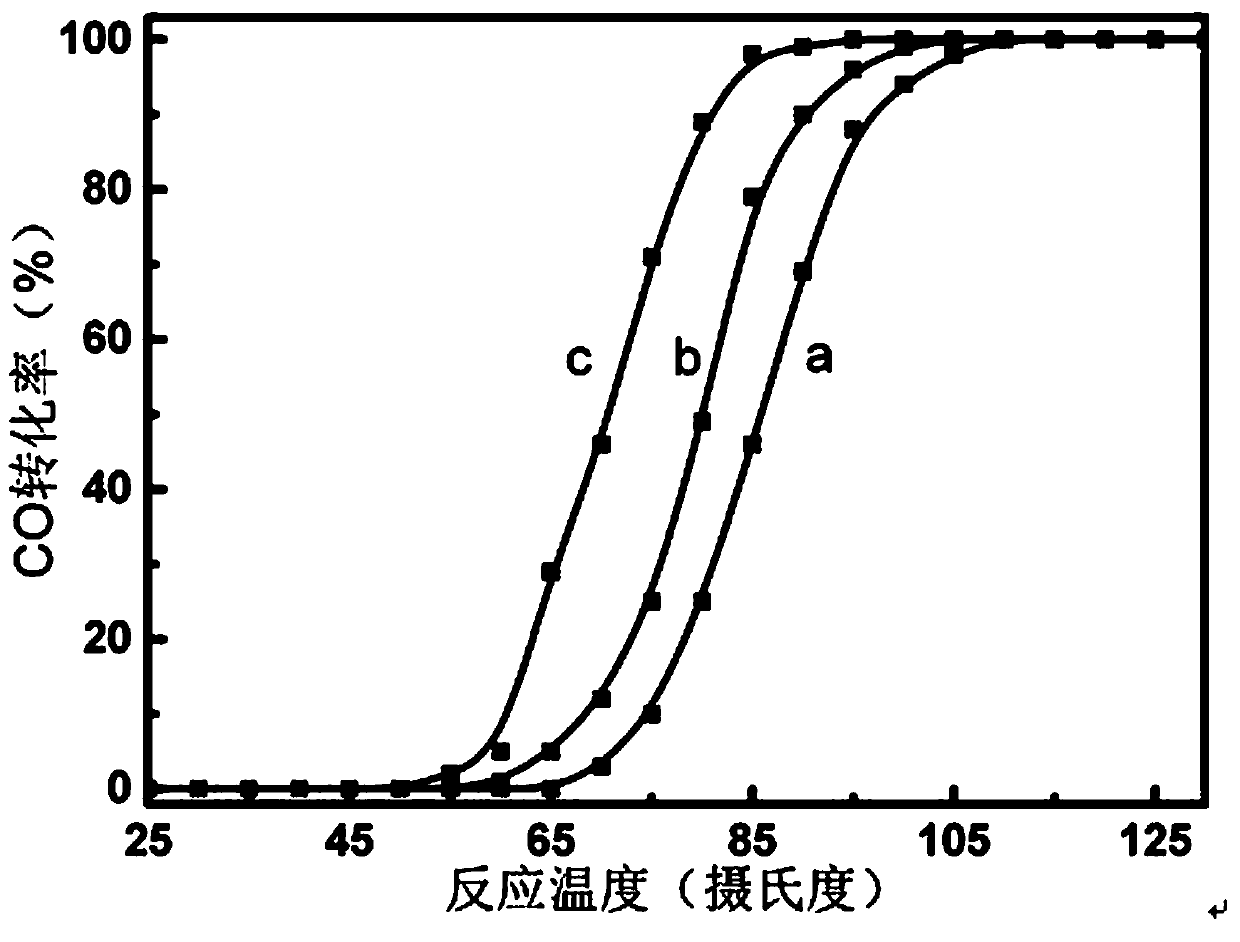
Image
Examples
Embodiment 1
[0019] (1) Weigh 3.75g of aluminum nitrate nonahydrate and 10g of polyethylene glycol 400, and add them to a polytetrafluoroethylene reaction vessel with a capacity of 50mL; seal the polytetrafluoroethylene reaction vessel and place it under constant temperature magnetic stirring at 80°C In the oil bath, continue magnetic stirring for 1 hour to obtain a molten mixture;
[0020] (2) Add 1 g of cetyltrimethylammonium bromide to the polytetrafluoroethylene reaction vessel, and continue magnetic stirring for 1 hour to obtain a melt-dispersed mixture;
[0021] (3) Quickly transfer the molten and dispersed mixture to a corundum crucible, put it into a tube furnace and calcinate at 800°C for 5 hours in a static air atmosphere, and obtain powdered alumina after cooling with the furnace.
Embodiment 2
[0023] (1) Weigh 4.04g of iron (III) nitrate nonahydrate and 10g of polyethylene glycol 400, and add them to a polytetrafluoroethylene reaction vessel with a capacity of 50mL; seal the polytetrafluoroethylene reaction vessel and place it in a 60°C In the constant temperature magnetic stirring oil bath, the magnetic stirring was continued for 1 hour to obtain the molten mixture liquid;
[0024] (2) Add 1 g of cetyltrimethylammonium bromide to the polytetrafluoroethylene reaction vessel, and continue magnetic stirring for 1 hour to obtain a melt-dispersed mixture;
[0025] (3) Quickly transfer the molten and dispersed mixture to a corundum crucible, put it into a tube furnace and calcinate at 700°C for 5 hours in a static air atmosphere, and obtain powdered iron oxide after cooling with the furnace.
Embodiment 3
[0027] (1) Weigh 2.91g of cobalt(II) nitrate hexahydrate and 10g of polyethylene glycol 600, and add them to a polytetrafluoroethylene reaction vessel with a capacity of 50mL; seal the polytetrafluoroethylene reaction vessel and place it in a 60°C In the constant temperature magnetic stirring oil bath, the magnetic stirring was continued for 1 hour to obtain the molten mixture liquid;
[0028] (2) Add 1 g of cetyltrimethylammonium bromide to the polytetrafluoroethylene reaction vessel, and continue magnetic stirring for 1 hour to obtain a melt-dispersed mixture;
[0029] (3) Quickly transfer the molten and dispersed mixture to a corundum crucible, put it into a tube furnace and calcinate at 600°C for 5 hours in a static air atmosphere, and obtain powdered cobalt tetroxide after cooling with the furnace.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com