Method for preparing metal oxides or metal composite oxides
A metal composite and oxide technology, applied in the direction of oxide/hydroxide preparation, metal/metal oxide/metal hydroxide catalyst, rare earth metal oxide/hydroxide, etc., can solve the difficult control of co-precipitation conditions And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
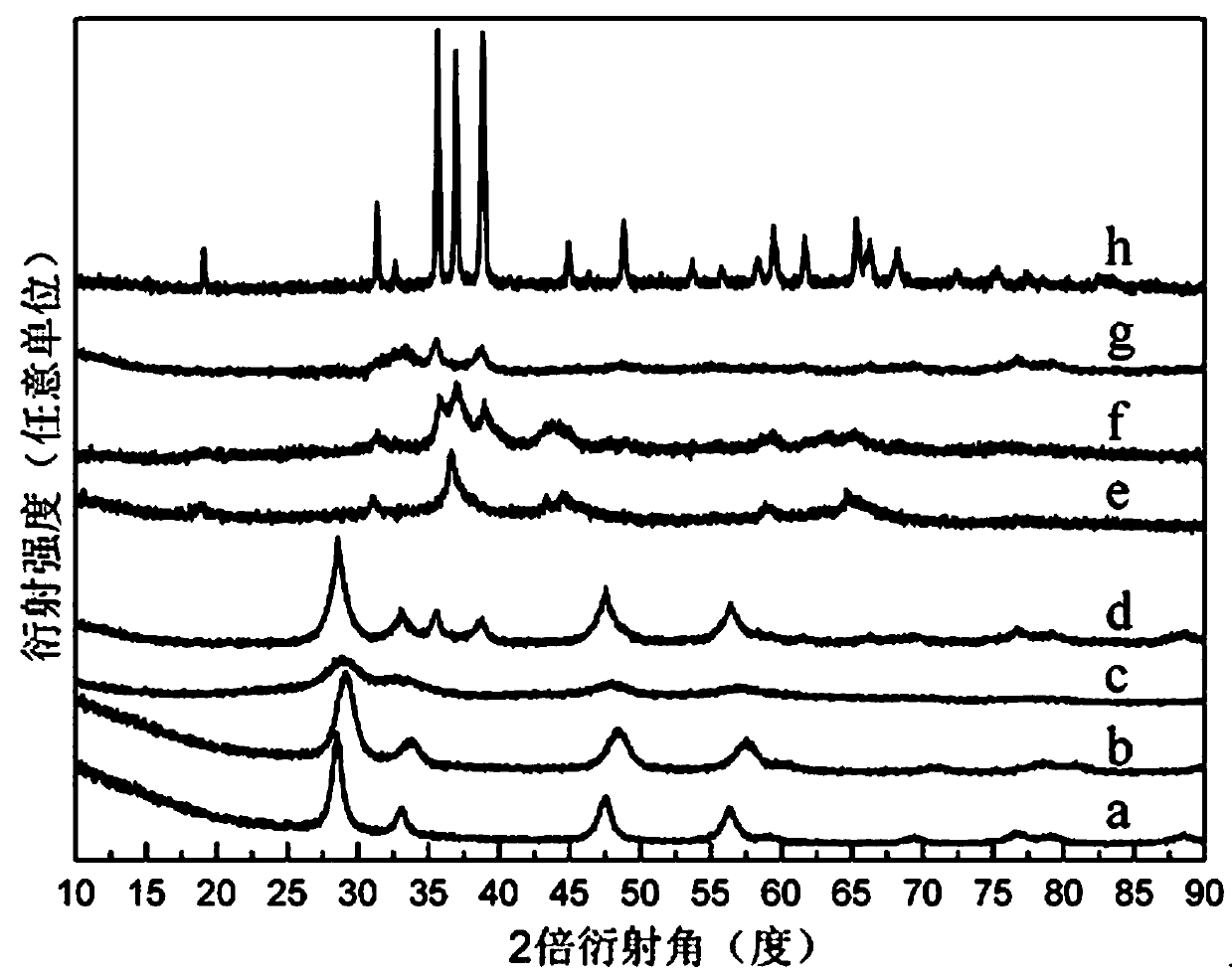
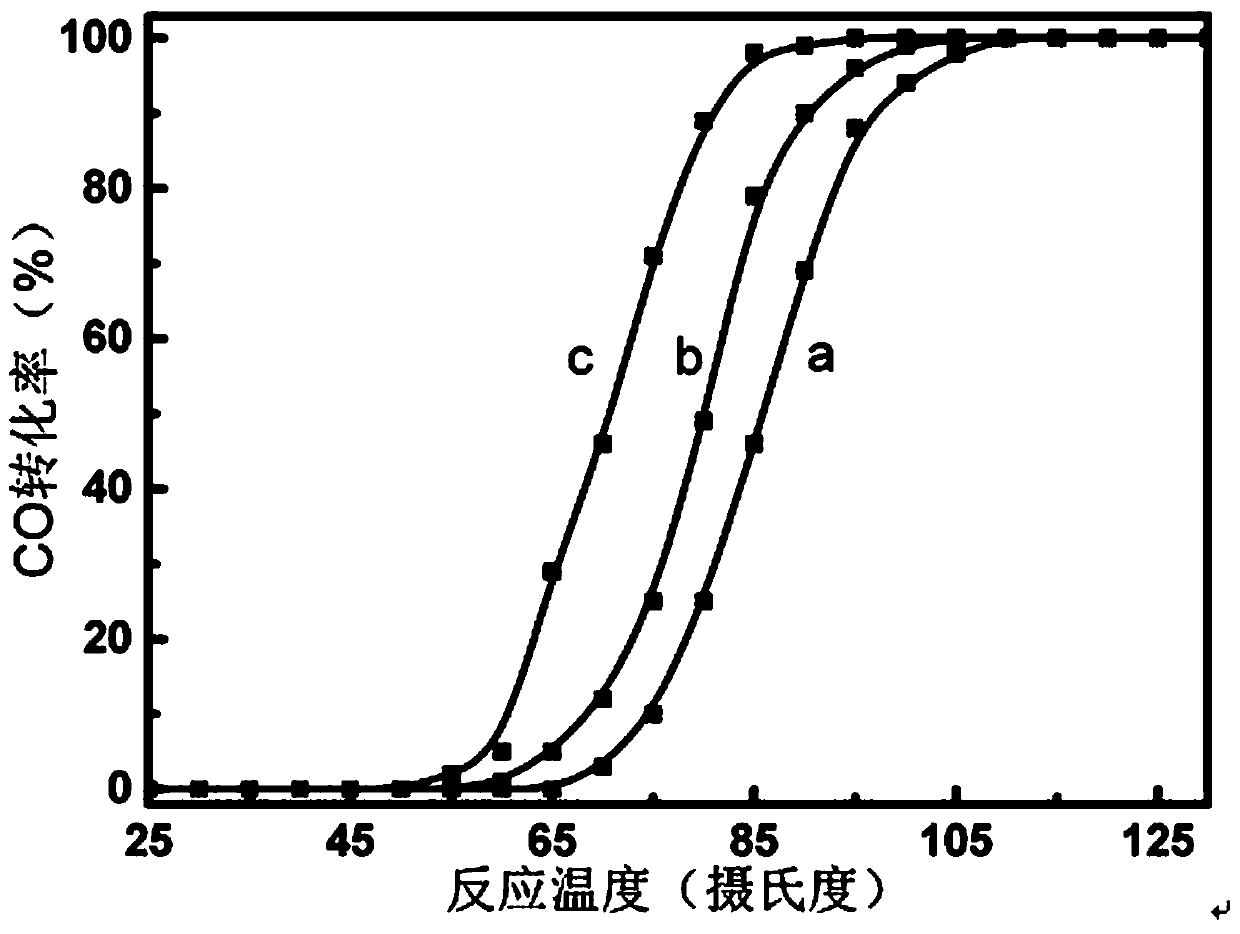
Image
Examples
Embodiment 1
[0019] (1) Weigh 3.75g of aluminum nitrate nonahydrate and 10g of polyethylene glycol 400, and add them to a 50mL PTFE reaction vessel; seal the PTFE reaction vessel and place it under constant temperature magnetic stirring at 80°C In the oil bath, magnetic stirring was continued for 1 hour to obtain a molten mixed liquid;
[0020] (2) Add 1 g of cetyl trimethyl ammonium bromide to the polytetrafluoroethylene reaction vessel, and continue magnetic stirring for 1 hour to obtain a melted and dispersed mixture;
[0021] (3) Quickly transfer the melted and dispersed mixture into a corundum crucible, and put it into a tube furnace for calcination at 800°C for 5 hours in a static air atmosphere, and cool down with the furnace to obtain powdered alumina.
Embodiment 2
[0023] (1) Weigh 4.04g of iron (III) nitrate nonahydrate and 10g of polyethylene glycol 400, and add them to a 50mL PTFE reaction vessel; seal the PTFE reaction vessel and place it in a 60°C In the constant temperature magnetic stirring oil bath, the magnetic stirring is continued for 1 hour to obtain the molten mixed material liquid;
[0024] (2) Add 1 g of cetyl trimethyl ammonium bromide to the polytetrafluoroethylene reaction vessel, and continue magnetic stirring for 1 hour to obtain a melted and dispersed mixture;
[0025] (3) Quickly transfer the melted and dispersed mixture into a corundum crucible, and put it into a tube furnace for calcination at 700°C for 5 hours in a static air atmosphere, and cool down with the furnace to obtain powdered iron oxide.
Embodiment 3
[0027] (1) Weigh 2.91 g of cobalt(II) nitrate hexahydrate and 10 g of polyethylene glycol 600, and add them to a 50 mL PTFE reaction vessel; seal the PTFE reaction vessel and place it in a 60°C In the constant temperature magnetic stirring oil bath, the magnetic stirring is continued for 1 hour to obtain the molten mixed material liquid;
[0028] (2) Add 1 g of cetyl trimethyl ammonium bromide to the polytetrafluoroethylene reaction vessel, and continue magnetic stirring for 1 hour to obtain a melted and dispersed mixture;
[0029] (3) Quickly transfer the melted and dispersed mixture into a corundum crucible, and put it into a tube furnace for calcination at 600 °C for 5 hours in a static air atmosphere, and then cool with the furnace to obtain powder cobalt tetroxide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com