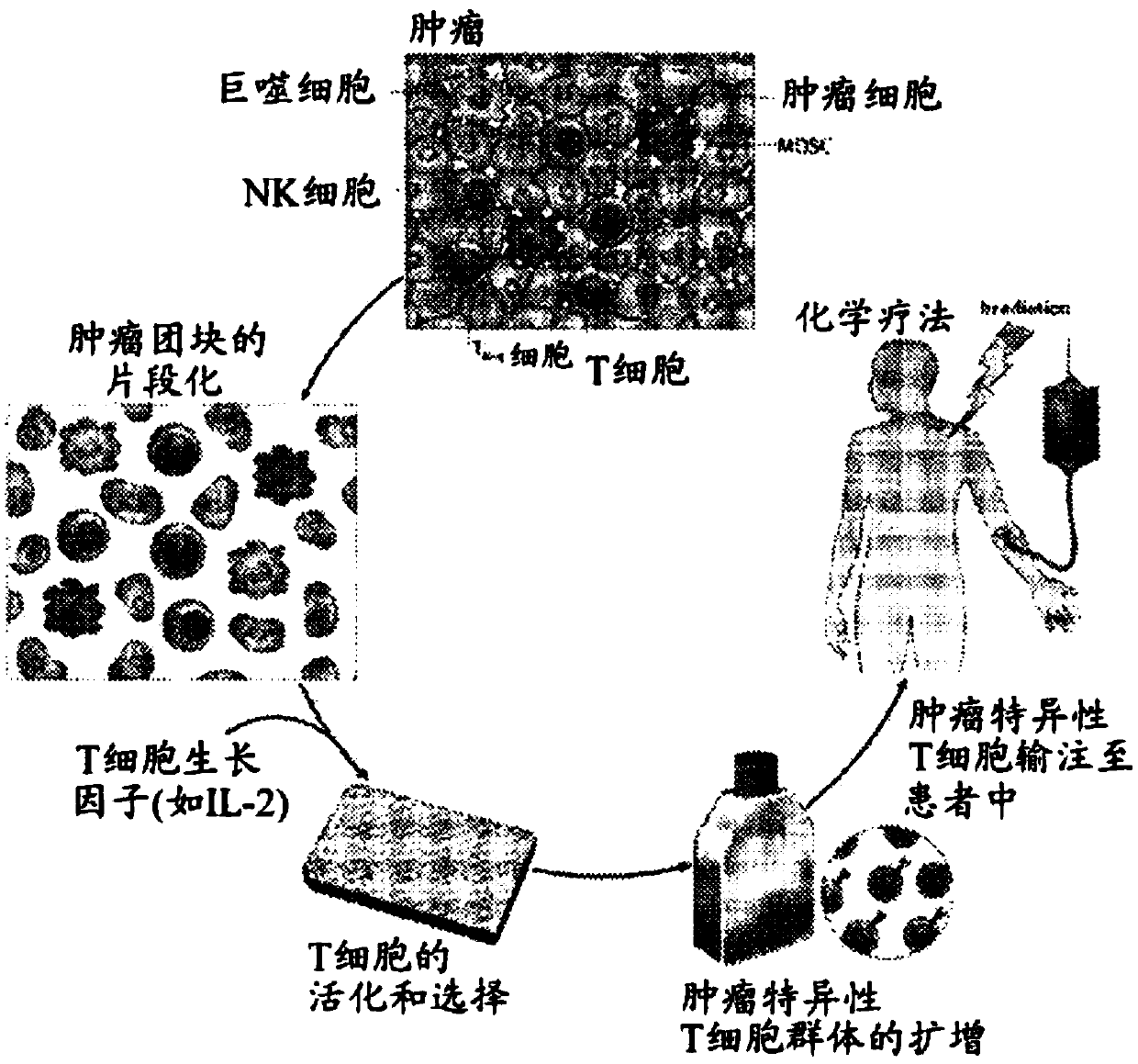
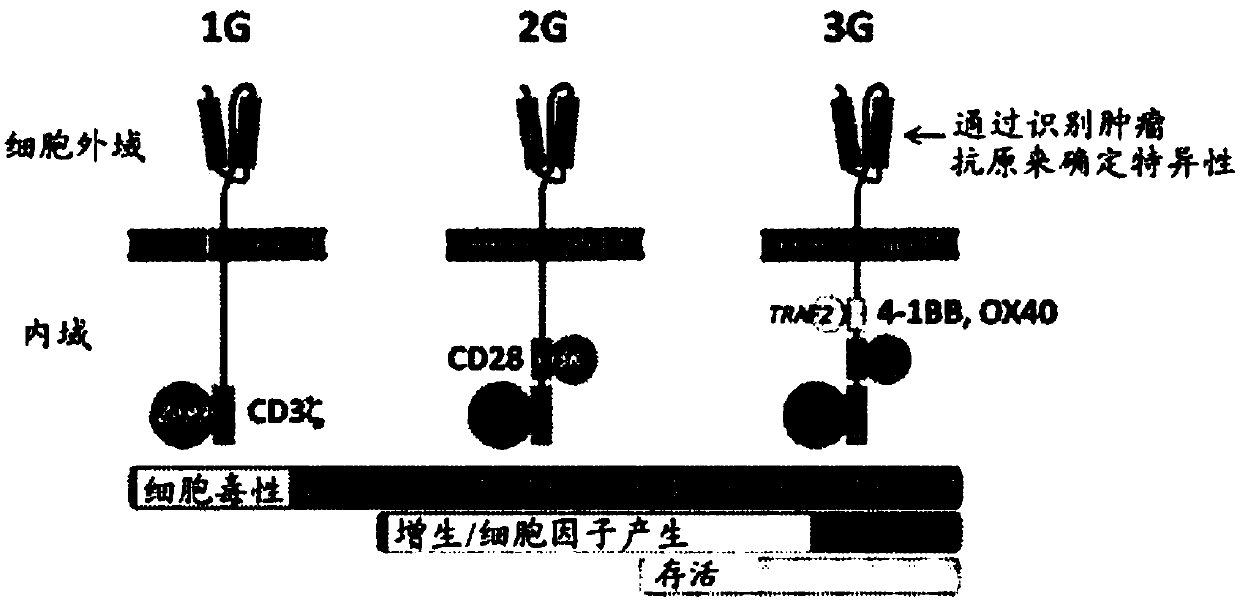
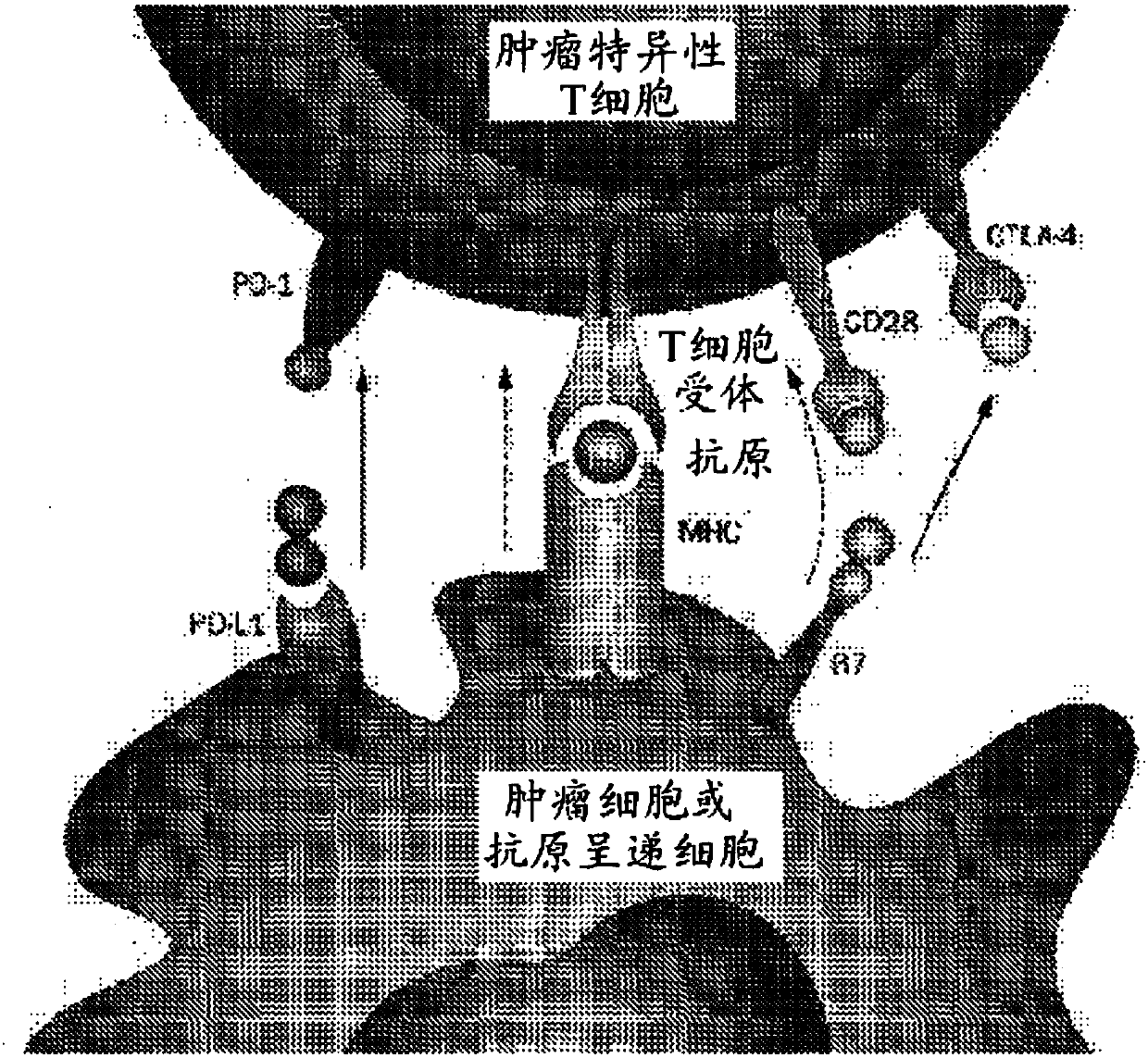
Modified t cells and methods of making and using the same
A cell and lymphocyte technology, applied in the field of modified T cells and their preparation and use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0310] 教导以上含磷键的制备的代表性美国专利包括但不限于美国专利号3,687,808;4,469,863;4,476,301;5,023,243;5,177,195;5,188,897;5,264,423;5,276,019;5,278,302;5,286,717;5,321,131;5,399,676;5,405,939;5,453,496;5,455,233;5,466,677 ;5,476,925;5,519,126;5,536,821;5,541,316;5,550,111;5,563,253;5,571,799;5,587,361;5,625,050;6,028,188;6,124,445;6,160,109;6,169,170;6,172,209;6,239,265;6,277,603;6,326,199;6,346,614;6,444,423;6,531,590;6,534,639;6,608,035;6,683,167;6,858,715;6,867,294 6,878,805; 7,015,315; 7,041,816; 7,273,933; 7,321,029; and US Patent RE39464, each of which is incorporated herein by reference in its entirety.
[0311] Modified internucleoside linkages in which the phosphorus atom is not included have short chain alkyl or cycloalkyl internucleoside linkages, mixed heteroatoms and alkyl or cycloalkyl internucleoside linkages, or one or more short chain Internucleoside linkages formed by heteroatom or heterocyclic internucleoside linkages. These include those with morpholino linkages (formed in part fro...
Embodiment 1
[0346] Example 1: Identification of CRISPR guide ribonucleic acid (gRNA) that efficiently removes endogenous TCR from primary human T cells to prevent autoreactivity of CART cells.
[0347] Prevention of autoreactivity has the highest priority to improve existing T cell-based therapies. The present invention aims to develop gRNAs with high on-target and no / low off-target activity that allow efficient and safe deletion of the endogenous T-cell receptor (TCR) in primary human T cells. The inventors designed multiple gRNAs targeting the TCR α and β chain loci (TRA and TRB) located on chromosomes 14 and 7, respectively ( Figure 10 ). Each gRNA first uses SURVEYOR TM Assays (data not shown) were tested for the ability to direct site-specific mutations in HEK293T cells. Then by flow cytometry (FACS) and SURVEYOR TM The gRNA candidate with the highest on-target efficiency was analyzed to assess functional TCR depletion in Jurkat T cells (FIG. 11A and FIG. 11B). Directions are d...
Embodiment 2
[0348] Example 2: Allogeneic T cells for CAR-T therapy.
[0349] T cell-based therapies are currently limited to the infusion of autologous cells obtained from the same patient. Extending the origin of T cells to allogeneic sources by generating universally applicable cell products will not only greatly reduce costs, but also open the door to a wider range of patients for this novel and promising class of therapies.
[0350] Complete loss of MHC class I surface expression can be achieved using CRISPR gRNA targeting the gene encoding accessory strand β2 microglobulin (B2M). The present inventors have recently identified gRNAs targeting B2M with extremely high on-target efficiency, which may have been adapted for use in gene therapy (Mandal et al., "Efficient Ablation of Genes in Human Hematopoietic Stem and Effector Cells using CRISPR / Cas9" Cell Stem Cell, 15:5, 643-652 (2014); Meissner et al., "Genome editing for human gene therapy," Methods inenzymology, 546, 273-295 (2014);...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com