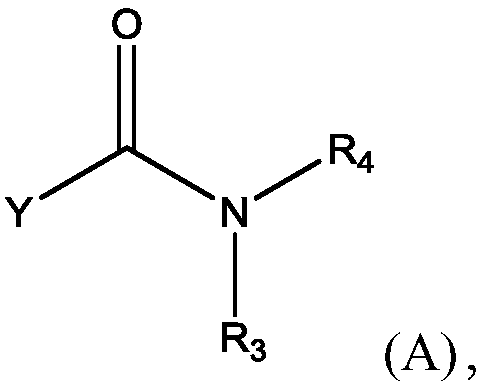
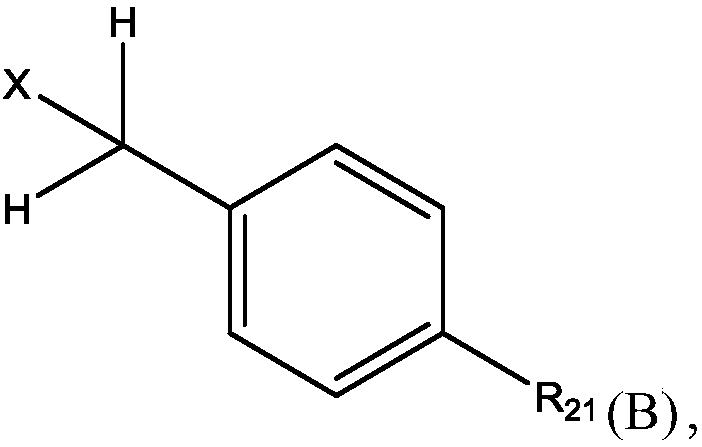
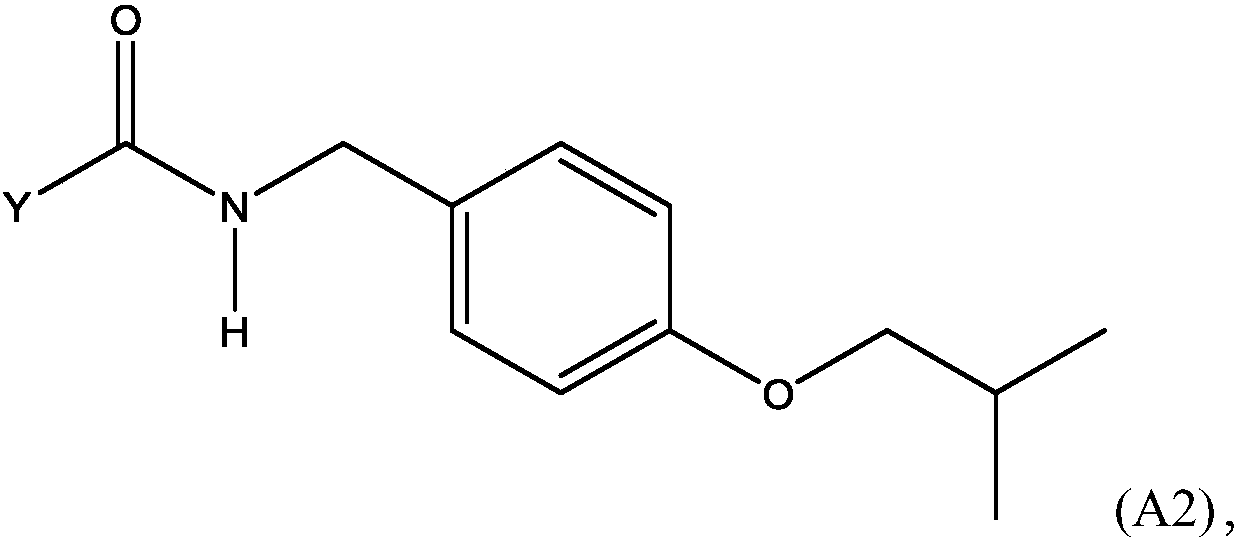
Methods for preparing n-(4-fluorobenzyl)-n-(1-methylpiperidin-4-yl)-n'-(4-(2-methylpropyloxy)phenylmethyl)carbamide and its tartrate salt and polymorphic form c
A kind of fluorophenylmethyl, methyl propoxy technology, applied in the field of medicine and chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0250] Experimental Procedures and Instruments
[0251] Examples of devices and methods useful in assisting in the preparation of the compounds described herein are:
[0252] NMR equipment: VARIAN INOVA 400mhz.
[0253] GC-MS equipment: TRACE GC THERMO FINNIGAN (method: column: ZB-5MS, 30x 0.25mm, 0.25μm. Temperature gradient: 50°C for 2min, then rise to 320°C at 20°C / min. Hold time: 15min. Flow rate : 1.0ml / min, split: 1:50, injection temperature: 200°C. injection volume: 1μL. diluent: acetonitrile).
[0254] LC-MS equipment: AGILENT 6530ACCURATE-MASS Q-TOF (Method: Column: ACQUITYUPLC BEHC18, 100x 2.1mm, 1.7μm. Eluent A: 0.1% HFo in acetonitrile. Eluent B: 0.1% HFo in h 2 O middle. Gradient: 10% A 90% B at 0 min, 95% A 5% B at 15 min, 95% A 5% B at 20 min. Flow rate: 0.25ml / min, temperature: 40°C. Injection volume: 1 μL. Diluent: Acetonitrile. ); or LCQ ADVANTAGE THERMO FINNIGAN (Method: Column: Symmetry Shield RP18, 150 x 3.0 mm, 3.5 μm, Eluent A: 0.05% TFA in Ace...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com