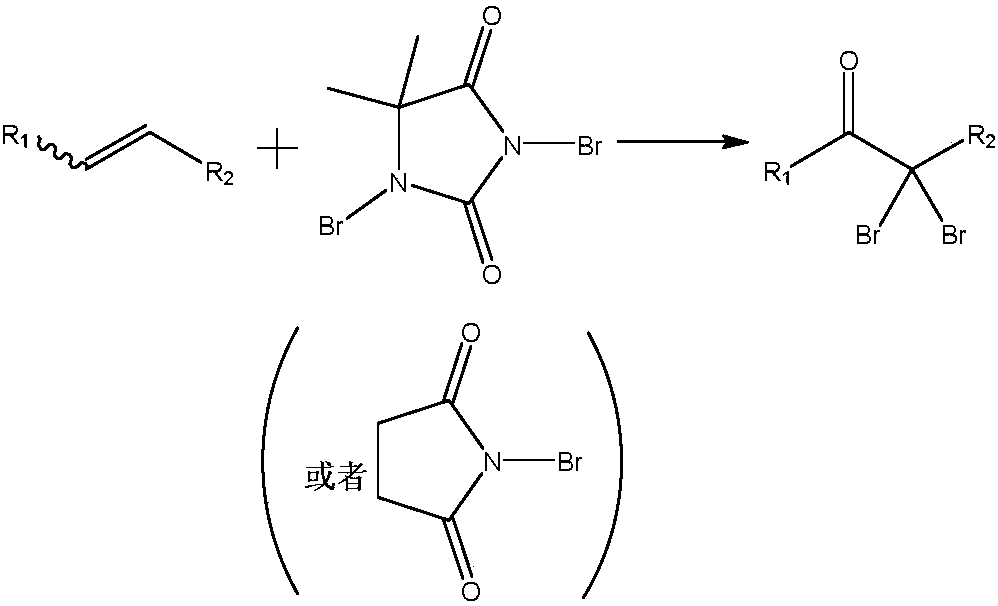
Preparation method of alpha, alpha-dibromoketone
A technology of dibromohydantoin and dibromohydantoin, which is applied in the preparation of organic compounds, carbon-based compounds, heterocyclic compounds, etc., can solve the problems of low yield and inconvenient operation, and achieve easy-to-obtain raw materials and easy operation , low-cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Preparation of 2,2-dibromoacetophenone
[0023] Using styrene as raw material, prepared according to the general method, the yield is 87%, and the NMR spectrum data of the product are as follows: 1 HNMR (400MHz, CDCl 3 )δ8.10–8.05 (m, 2H), 7.64 (t, J = 7.4Hz, 1H), 7.51 (t, J = 7.7Hz, 2H), 6.72 (s, 1H).
Embodiment 2
[0024] Example 2 Preparation of 2,2-dibromo-2'-methylacetophenone
[0025] Using o-methyl styrene as raw material, it is prepared according to the general method, and the yield is 70%. The NMR spectrum data of the product are as follows: 1 HNMR (400MHz, CDCl 3 ) δ 7.68 (d, J = 7.6Hz, 1H), 7.46 (t, J = 7.3Hz, 1H), 7.34–7.28 (m, 2H), 6.68 (s, 1H), 2.52 (s, 3H).
Embodiment 3
[0026] Example 3 Preparation of 2,2-dibromo-4'-methylacetophenone
[0027] Taking p-methylstyrene as raw material, it is prepared according to the general method, and the yield is 80%. The NMR spectrum data of the product are as follows: 1 HNMR (400MHz, CDCl 3 ) δ 7.98 (d, J = 8.3Hz, 2H), 7.31 (d, J = 8.0Hz, 2H), 6.70 (s, 1H), 2.44 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com