A kind of oxygen evolution reaction electrocatalyst, its preparation method and application
An oxygen evolution reaction and electrocatalyst technology, which is applied in the field of preparation of β-Ni2 nanorods, can solve the problems of high overpotential, high price, complex synthesis method, etc., and achieves the effects of good OER activity, convenient operation and simple equipment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
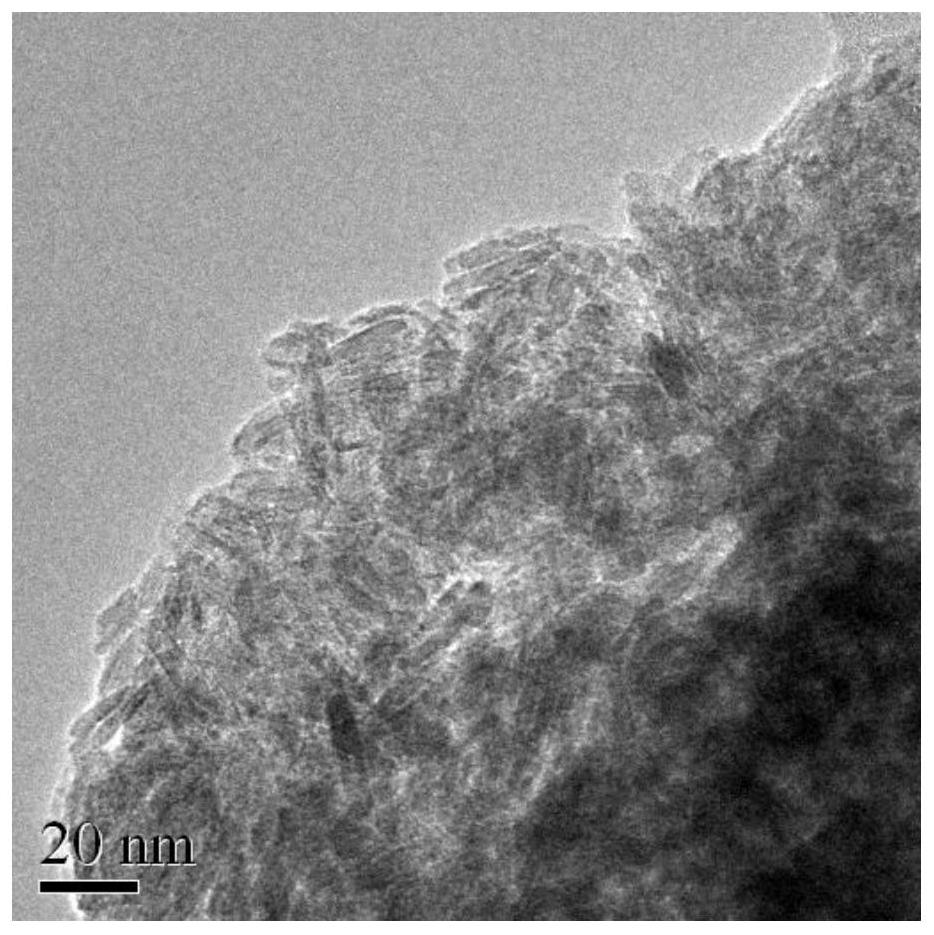
[0013] The invention provides a preparation method of an oxygen evolution reaction electrocatalyst and a catalyst prepared thereby. The oxygen evolution reaction electrocatalyst is Fe 3+ Doped β-Ni(OH) 2 .
[0014] The present invention firstly provides a preparation method of an oxygen evolution reaction electrocatalyst. The present invention is based on an improved atomic-level topological chemical conversion method. Under the protection of an inert gas, the divalent Fe-doped Doped β-Ni(OH) 2 , and then oxidized in an oxidative atmosphere by atomic-level topological chemical conversion method to generate trivalent Fe-doped β-Ni(OH) 2 catalyst.
[0015] The above-mentioned method of the present invention comprises the steps of mixing the nickel-iron salt solution and the alkali solution, and exposing the mixed system to an oxidizing atmosphere.
[0016] Wherein, the ferrous nickel salt solution is a solution obtained by dissolving soluble ferrous salt and soluble nickel ...
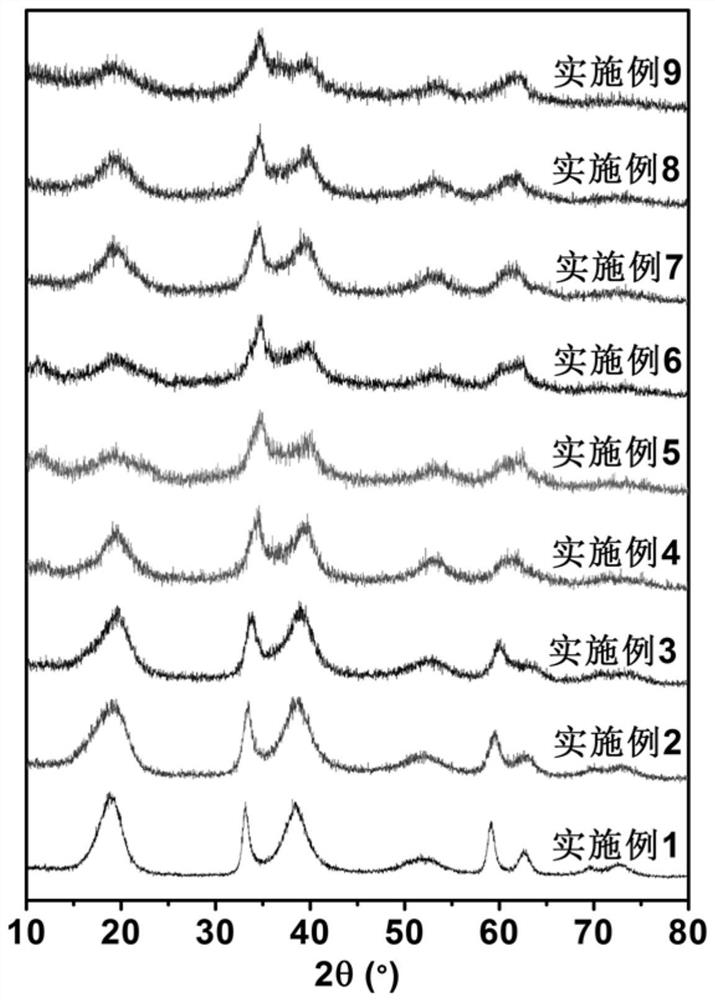
Embodiment 1
[0038] Weigh 2.7801 grams of nickel sulfate hexahydrate and dissolve it in 20 milliliters of water, the concentration of nickel sulfate is 0.5 moles per liter. Under the protection of nitrogen atmosphere, 20mL of 0.5mol / L nickel sulfate solution was added dropwise into 80mL of 1.25mol / L potassium hydroxide solution, and the dropwise addition was completed in 20min. The reaction temperature was 80°C. Then the cloudy system was exposed to the air and reacted for 5h. Finally, it was washed with water until pH = 7, washed three times with absolute ethanol, and then centrifuged. Disperse the product in ethanol, and dry it in a low-temperature vacuum in a freeze dryer. A green powder was obtained. Weigh the obtained green powder and 5 mg of XC-72 and disperse in 2 mL of isopropanol, then add 50 μL of Nafion (5%) solution, and ultrasonically shake for 30 min to mix evenly to obtain a catalyst slurry. Then pipette 20 μL of the above catalyst slurry onto a rotating disk electrode wi...
Embodiment 2
[0040] Weigh 0.2628 grams of ferrous sulfate heptahydrate and 2.5021 grams of nickel sulfate hexahydrate and dissolve them in 20 milliliters of water. The total concentration of ferrous sulfate and nickel sulfate is 0.5 moles per liter. Under the protection of nitrogen atmosphere, 20mL of 0.5mol / L nickel sulfate solution was added dropwise into 80mL of 1.25mol / L potassium hydroxide solution, and the dropwise addition was completed in 20min. The reaction temperature was 80°C. Then the cloudy system was exposed to the air and reacted for 5h. Finally, it was washed with water until pH = 7, washed three times with absolute ethanol, and then centrifuged. Disperse the product in ethanol, and dry it in a low-temperature vacuum in a freeze dryer. A yellow-brown powder was obtained. The obtained yellow-brown powder and 5 mg of XC-72 were weighed and dispersed in 2 mL of isopropanol, then 50 μL of Nafion (5%) solution was added, and ultrasonically oscillated for 30 min to mix uniforml...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com