Method for biosynthesis of D-tagatose by immobilized enzyme catalyst
An immobilized enzyme and biosynthesis technology, applied in the field of bioengineering, can solve the problems of low conversion rate of D-tagatose, poor stability, low activity of L-arabinose isomerase, etc., and achieve improved conversion rate and efficient production. , the effect of improving vitality and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Spore Display L-AI Recombinant Vector and Construction of Recombinant Spore
[0031] 1. According to the characteristics of the multiple cloning site on the araA gene sequence of Lactobacillus brevis and the carrier pHS (no antibiotic gene), use bioinformatics software to design synthetic primers: primer 1, 5'- GGTGGCGGCGGTTCTGGTGGCGGCGGTTCT CTAAAGGAGTGCTCAATTATG-3'; Primer 2, 5'- CGCTCTAGAACTAGT CCATTTTAAATGTTTAGC. The sequence of the underlined part in primer 1 encodes Gly-Gly-Gly-Gly-Ser-Gly-Gly-Gly-Gly-Ser (Linker), and the sequence of the underlined part in primer 2 is linear with EcoRI and BamHI double digestion One end of the pHS plasmid is complementary.
[0032] 2. Use Lactobacillus brevis genomic DNA as a template to amplify the target gene by PCR.
[0033] 3. PCR reaction parameters: pre-denaturation, 95°C for 5min; denaturation at 94°C for 2min, annealing, 55°C for 30s, extension, 72°C for 90s, after 35 cycles, keep at 72°C for 10min. Finally, keep war...
Embodiment 2
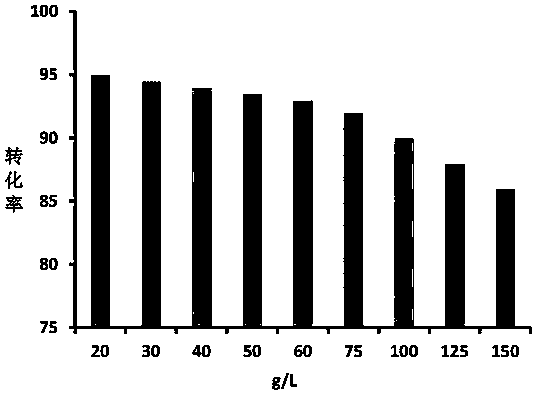
[0045] Conversion of 20g / L D-galactose to D-tagatose with L-arabinose isomerase immobilized enzyme catalyst
[0046] 1. Resuspend the recombinant Bacillus subtilis spores in phosphate buffer solution with pH 6.5 to make a concentration of 0.78×10 9 The recombinant Bacillus subtilis spore suspension of spore / mL is standby; Then 40mL (0.78×10 9 Spores / mL) Recombinant Bacillus subtilis spore suspension was added in 400mL reaction solution;
[0047] 2. Under the conditions of 70°C and 220rpm, shake and transform in a 2L shake flask for 24 hours, take samples from time to time, and obtain D-tagatose in the transformation solution;
[0048] 3. Centrifuge at 8000rpm and collect the recombinant Bacillus subtilis spores in step 2 and add them to 400mL reaction solution;
[0049] 4. Repeat steps 2 and 3 4 times in sequence to calculate the conversion rate and residual activity of the immobilized enzyme catalyst.
[0050] Wherein the reaction liquid composition described in step 1 is ...
Embodiment 3
[0054] Conversion of 150g / L D-galactose to D-tagatose with L-arabinose isomerase immobilized enzyme catalyst
[0055] 1. Mix 40mL (0.78×10 9 Spores / mL) Recombinant Bacillus subtilis spore suspension was added in 400mL reaction solution;
[0056] 2. Under the conditions of 70°C and 220rpm, shake and transform in a 2L shake flask for 24 hours, take samples from time to time, and obtain D-tagatose in the transformation liquid;
[0057] 3. Centrifuge at 8000rpm and collect the recombinant Bacillus subtilis spores in step 2 and add them to 400mL reaction solution;
[0058] 4. Repeat steps 2 and 34 in sequence to calculate the conversion rate and residual activity of the immobilized enzyme.
[0059] Wherein the composition of the reaction solution described in step 1 is as follows: D-galactose 150g / L, MnCl 2 1mM, 100mM pH6.5 potassium phosphate buffer;
[0060] Wherein the enzyme activity assay method described in step 4 is as follows: in 1mL reaction volume, contain 500mM D-ga...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com