3,4,6-Trisubstituted-alpha-pyrone derivative and preparation method and application thereof
A technology of pyrone and derivatives is applied in the application field of preparing tomato gray mold inhibitor, and can solve problems such as drugs that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
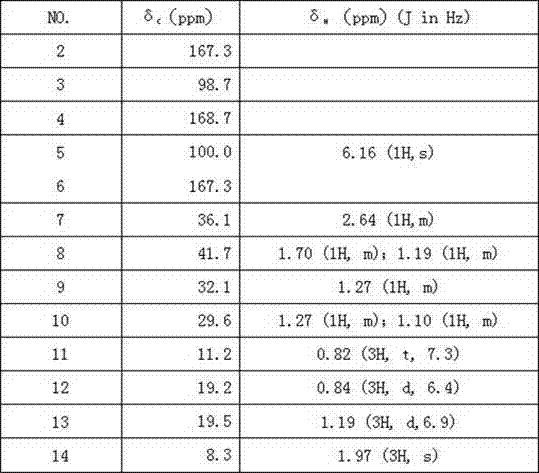
[0023] A 3,4,6 trisubstituted-α-pyrone derivative structural formula is shown in I:
[0024] (I).
Embodiment 2
[0026] The preparation method of 3,4,6 three substituted-alpha-pyrone derivatives as shown in formula I, specifically comprises the following steps:
[0027] (1) Fermentation production
[0028] Aspergillus versicolor ( Aspergillus versicolor DJ013) was revived by streaking, transferred to a 250mL Erlenmeyer flask containing 100mL PDB medium, and cultured at 28°C and 180 r / min for 4 days to obtain a seed culture solution, and then the inoculum was inoculated with a volume ratio of 10%. The seed solution was inoculated into a 1000mL Erlenmeyer flask containing rice culture medium (prepared by dissolving 80g rice and 1g yeast powder in 120mL seawater), and cultured at 28°C for 35 days to obtain a fermented product;
[0029] (2) Obtaining the extract
[0030] Soak the above-mentioned fermented product in 4L of methanol for 3 times, then concentrate and evaporate the methanol extract to dryness, redissolve it in 1L of water, repeat the extraction 3 times with 1L of ethyl acetate...
Embodiment 3
[0038] In vitro anti-Botrytis cinerea test (96-well plate antibacterial method)
[0039] (1) Experimental samples
[0040] Preparation of the test sample solution: the test sample is the pure compound I isolated and purified in Example 1 above, and an appropriate amount of sample is accurately weighed, and prepared into a solution of the required concentration with DMSO for testing the activity.
[0041] (2) Experimental method
[0042] 96-well plate method: prepare 1 mg / mL DMSO sample solution of the compound to be tested, and in a 96-well plate, use sterilized PDB liquid medium to dilute to concentrations of 200, 100, 50, 25, 12.5, 6.25 μg / mL solution (50μL), add an equal amount of 1×10 6 CFU bacterial suspension, the final concentration is: 200, 100, 50, 25, 12.5, 6.25, 3.13μg / mL, shake well, and all the above operations are under sterile conditions. DMSO was used as blank control and carbendazim was used as positive control, and each treatment was repeated 3 times. The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com