Alkali-sensitive ring opening cucurbituril and application thereof
A cucurbituril, sensitive technology, applied in the field of supramolecular drug carriers, to achieve the effect of increasing water solubility, strengthening host-guest bonding ability, and avoiding irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the synthetic preparation of tetramer
[0024] With reference to the method in the literature Ma D, et al. Nature Chemistry, 2012, 4(6):503:
[0025] (1) Synthesis of dimer: Mix glycoluril (500 g, 3.51 mol) and paraformaldehyde (105 g, 3.51 mol) evenly and add it to HCl solution (8 M, 700 mL). Heated for 48 hours; after the reaction was completed, the reaction solution was cooled and vacuum filtered to obtain a crude product; the crude product was washed with water (500 mL), and then recrystallized with TFA (1.5 L) to obtain a dimer as a white solid ( 334g, 62%);
[0026] ;
[0027] (2) Synthesis of tetramer: Dimer (304 g, 1.20 mol) was added to anhydrous MeSO 3 H (600 mL), after the dimer was completely dissolved and the solution became transparent, then etherified methyl glycoluril (84 g, 0.27 mol) was added; the reaction solution was reacted at 50°C for 3 hours; the reaction was completed Finally, the reaction solution was poured into water (6.0 L...
Embodiment 2
[0031] Embodiment 2: the synthesis of the side arm of benzene ring containing carboxylic acid group
[0032] Add N,N-dimethylformamide (approximately 10 mL) to 3 g (0.024 mol) of p-methoxyphenol, and add 3 mL of methyl bromopropionate after the p-methoxyphenol is completely dissolved. After fully stirring, add 4 g (0.029 mol) K 2 CO 3 , and reacted at room temperature for 20 h to generate p-methoxyphenylpropionyl methyl ester; the reaction solution was poured into methanol for recrystallization; the crude product was obtained after vacuum filtration; the crude product was washed three times with methanol (100 mL), and after suction filtration Dry with a vacuum oven to obtain p-methoxyphenylpropionyl methyl ester.
[0033] Take 3.2 g p-methoxyphenylpropionyl methyl ester, with 10 mL H 2 O and 10 mL tetrahydrofuran were used to dissolve the ester, and 3 g (0.125 mol) LiOH was slowly added to hydrolyze p-methoxyphenylpropionyl methyl ester; after the reaction, the reaction sol...
Embodiment 3
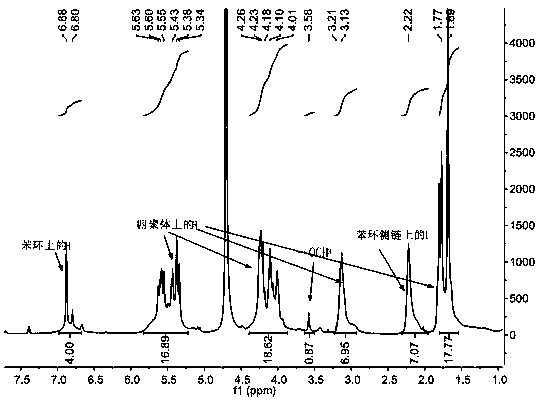
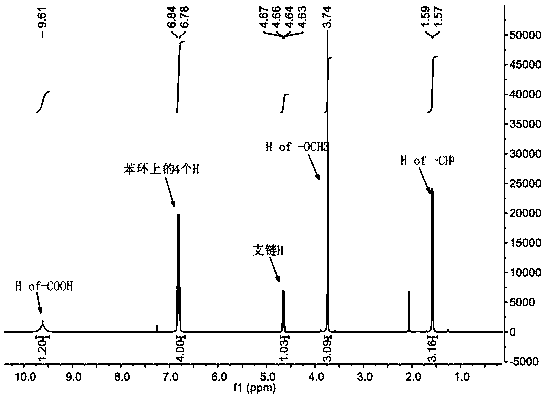
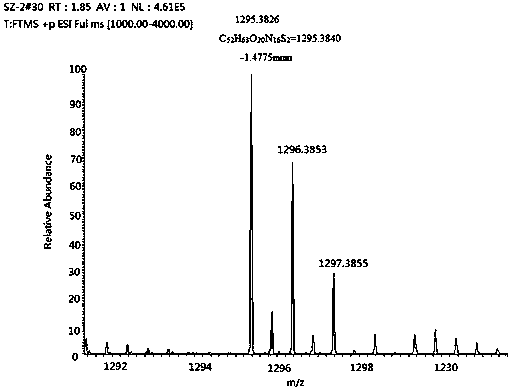
[0035] Example 3: Synthesis of Alkali Sensitive Open-ring Cucurbituril
[0036] Fully dissolve 1.8 g (2.45 mmol) of tetramer in 8 mL of acetic anhydride and 8 mL of trifluoroacetic acid, and mix thoroughly in an oil bath at 60°C. After the tetramer is completely dissolved, add two different The side arm of the benzene ring continued to react for 3 h; the molar ratio of the tetramer, the side arm of the benzene ring containing sulfonic acid groups, and the side arm of the benzene ring containing carboxylic acid groups was 1:1:1;
[0037] .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com