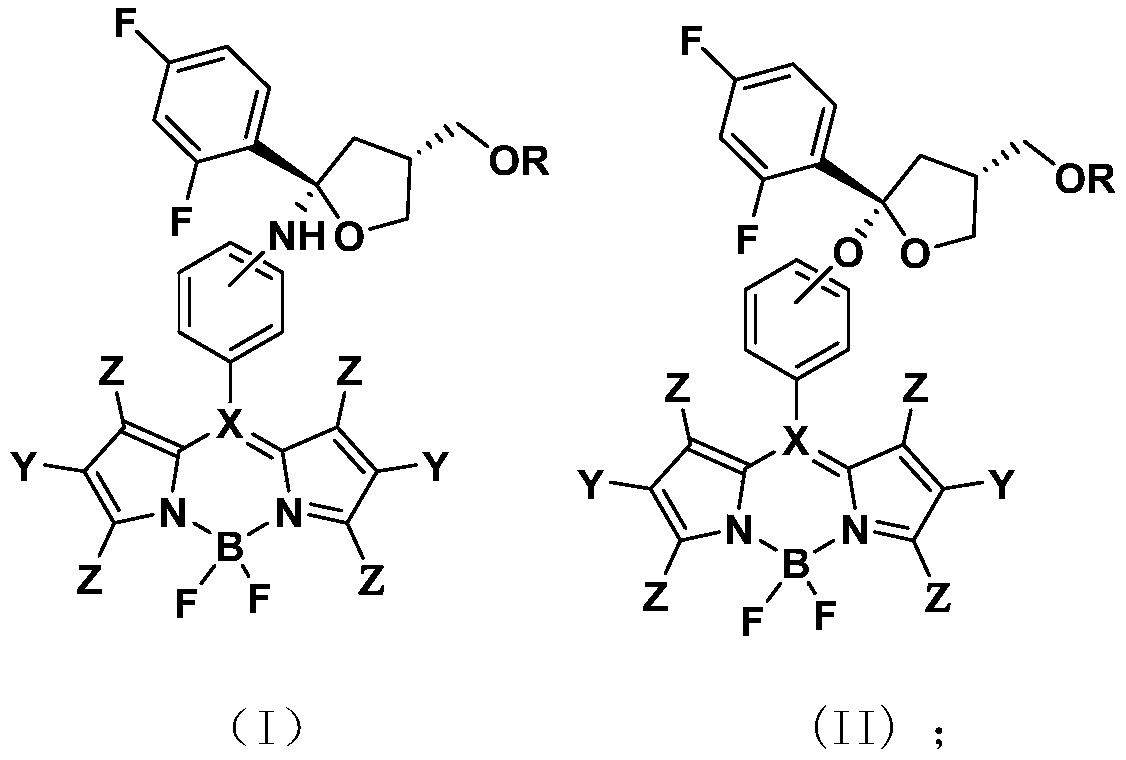
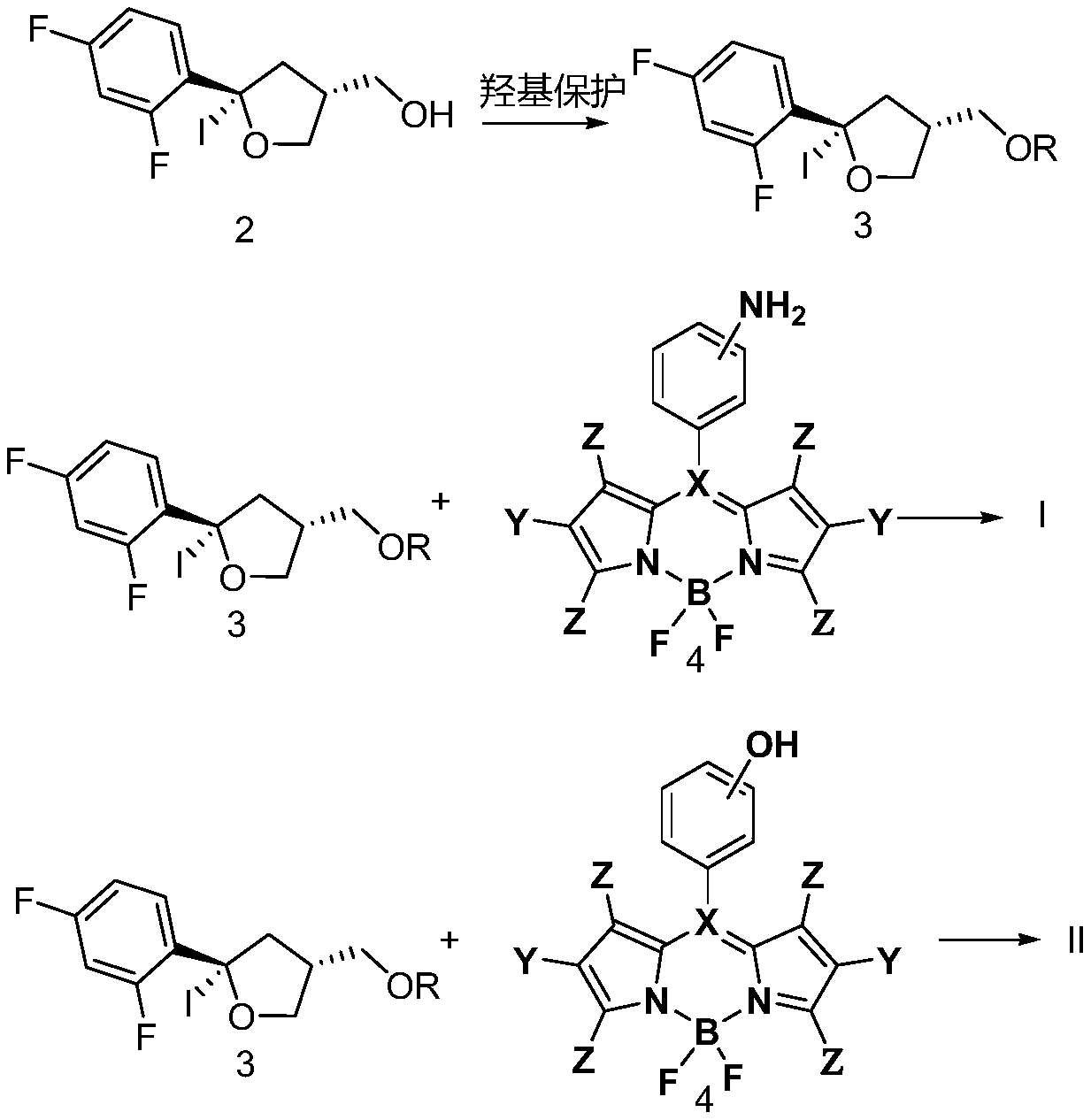
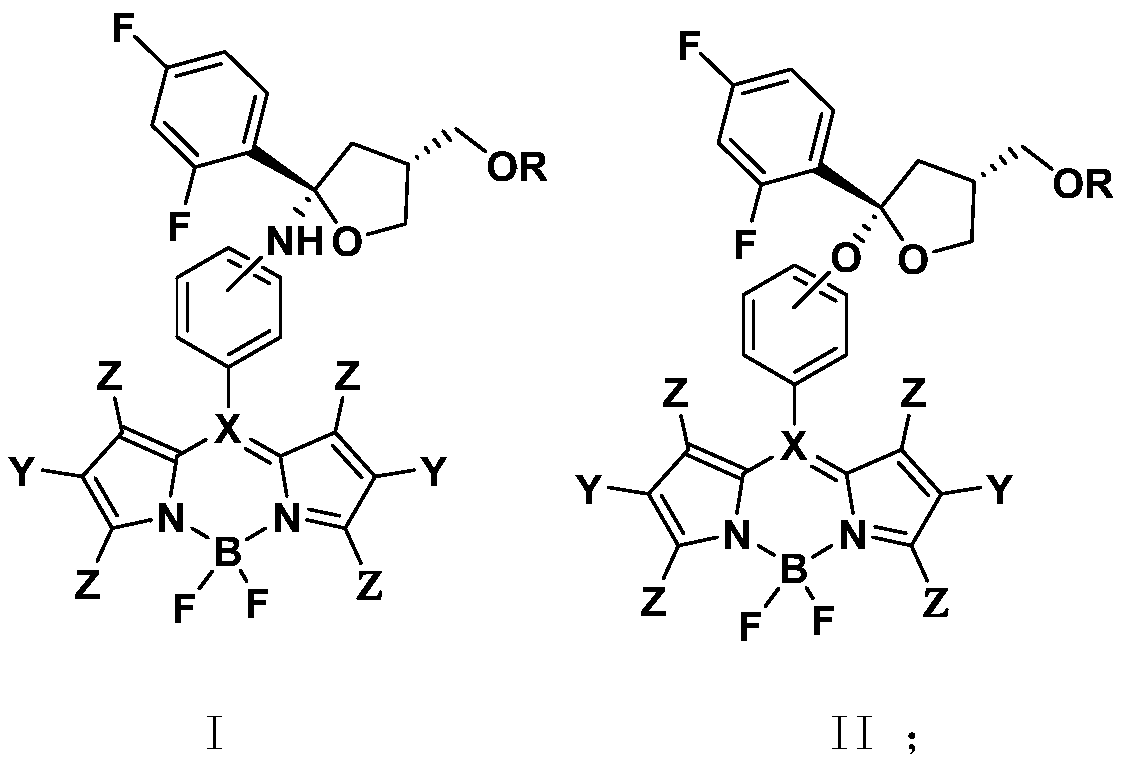
Chiral azadipyrrole and its application in the preparation of drugs for inhibiting pathogenic bacteria infection
A technology of heterodipyrrole and pathogenic bacteria, applied in the field of chiral azadipyrrole antibacterial drugs and its application, can solve the problems of life-threatening, high mortality and bacterial infection of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A kind of chiral azadipyrrole is made by the following method:
[0030] 1) Compound 2 (10 mmol, 3.4 g) was dissolved in CH 2 Cl 2 (50mL), add CH 3 OCH 2 Cl (12mmol, 0.8g), i- Pr 2 NEt (catalyst), at 25° C., reacted for 1 h, monitored by TLC, extracted and separated, and separated by column chromatography to obtain compound 3a (3.64 g, yield 95%).
[0031] 2) will 10mmol, 2.83g) was dissolved in THF (50mL), compound 3a (0.8mmol, 3.07g) was added, NaHCO 3 (10mmol, 0.84g), stirred at room temperature for 1h, monitored by TLC, extracted and separated, separated by column chromatography, and concentrated for later use;
[0032] 3) The concentrate of (2) obtained in the step was dissolved in methanol (50 mL), and a catalytic amount of HCl was added dropwise at 50° C. for 10 min, followed by extraction and separation, and column chromatography to obtain compound I-5 (4.71 g, 96% ). 1H NMR (300MHz, CDCl3)δ
[0033] 7.16-7.26(m, 2H), 7.11(d, J=8.7Hz, 1H), 6.78-6.86(m,...
Embodiment 2
[0035] A kind of chiral azadipyrrole is made by the following method:
[0036] 1) with Alternative to Example 1 Others are the same as in Example 1 to obtain compound I-6
[0037] (4.71 g, 96%).
Embodiment 3
[0039] A kind of chiral azadipyrrole is made by the following method:
[0040] 1) Compound 2 (10 mmol, 3.4 g) was dissolved in CH 2 Cl 2 (50mL), add CH 3 OCH 2 Cl (12mmol, 0.8g), i- Pr 2 NEt (catalyst), at 25° C., reacted for 1 h, monitored by TLC, extracted and separated, and separated by column chromatography to obtain compound 3a (3.64 g, yield 95%). ;
[0041] 2) will ( 10mmol, 2.83g) was dissolved in THF (50mL), compound 3a (0.8mmol, 3.07g) was added, NaHCO 3 (10 mmol, 0.84 g), stirred at room temperature for 1 h, monitored by TLC, extracted and separated, dried and concentrated, and separated by column chromatography to obtain compound I-8 (4.57 g, 80%).
[0042] 3) Dissolve the concentrate of (2) obtained in the step in methanol (50 mL), add dropwise a catalytic amount of HCl, 50° C., 10 min, extract and separate, and separate by column chromatography to obtain compound I-7 (4.85 g, 98% ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com