In vitro expansion method of NK cells and kit for method
A technology of NK cells and cells, which is applied in the direction of cell culture active agents, animal cells, vertebrate cells, etc., can solve the problems of troublesome safety, high cost and insufficient amplification methods, and achieve the effect of simple use and meeting clinical needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: In vitro expansion of NK cells in umbilical cord blood samples
[0029] 1. Melt 1.5 μg of HER-2 antibody (Tongli Haiyuan, product number: TL-503) as an antibody for inducing differentiation of NK cells, add it to 15ml of normal saline, mix well, and add it to the 175cm 2 The bottom area of the cell culture bottle, and the liquid is fully dispersed at the bottom of the bottle, and placed in a refrigerator at 4°C overnight.
[0030] 2. Aseptically collect 50ml of umbilical cord blood from healthy puerperas (collected from the Department of Obstetrics and Gynecology, Siping Hospital, China Medical University with the consent of the patient);
[0031] 3. Transfer the collected blood sample to a 50ml centrifuge tube (Corning, product number: 430828), and use a Thermo 4KR centrifuge at room temperature to adjust the speed to 800g, and centrifuge for 10 minutes;
[0032] 4. Absorb the upper layer of plasma for later use during cultivation; dilute the blood accord...
Embodiment 2
[0040] Example 2: Cell mass, amplification factor and viability after cell expansion
[0041] Total cell expansion factor: the cells obtained on the 7th, 14th, and 21st days of culture in the above examples were stained with trypan blue and then counted with a hemocytometer, and the current total cells were divided by the total number of mononuclear cells before culture , the value is the amplification factor of the cells, cell viability (%)=number of unstained cells / total number of observed cells×100. The results are shown in Table 1 and Figure 1A and Figure 1B , Figure 1A In the picture under the inverted microscope on the 7th day of culture, it can be seen that the cells are bright and in good condition, the proliferation spheres are scattered, and the cells are in the shape of irregular long sweet potatoes; Figure 1B The picture under the inverted microscope on the 14th day of culture shows that the cell density is significantly higher than that of the 7th day, and ...
Embodiment 3
[0044] Example 3: Detection of CD3 before and after cell expansion by flow cytometry - CD16 + CD56 + NK cell ratio
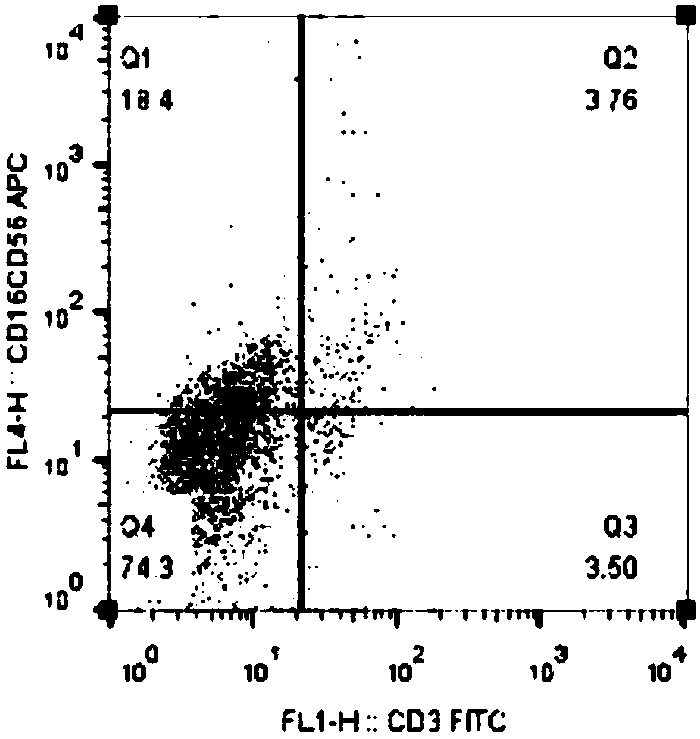
[0045] Flow cytometry detection method: Take the cell samples obtained after culturing the 7th day, 14th day and 21st day in Example 1 5×10 5 Cells / tube, wash 2 times with PBS. Add flow detection antibodies respectively for double-labeled flow phenotype detection: FITC-labeled mouse anti-human CD3 antibody (BioLegend, Cat. No. 300306) and APC-labeled mouse anti-human CD16 antibody (BioLegend, Cat. Product No. 304610); incubate at room temperature for 20 min, wash with PBS twice, and analyze the cells by flow cytometry.
[0046] The result is as Figure 2A to Figure 2C As shown, after 14 days of induction culture CD3 - CD16 + CD56 + About 18.4% of NK cells (Q1 area) on day 7 ( Figure 2A ) increased to about 87.8% ( Figure 2B ), CD3 was detected by flow cytometry after 21 days of culture - CD16 + CD56 + NK cells (Q1 area) increased to 96.8% ( Figu...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap