CD19 targeted chimeric antigen receptor, method of dual-modifying same, and application of the CD19 targeted chimeric antigen receptor
A receptor, single-chain antibody technology, applied in the field of chimeric antigen receptors targeting CD19, which can solve the problems of exceeding the therapeutic requirements, T cell attack, and high expansion volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
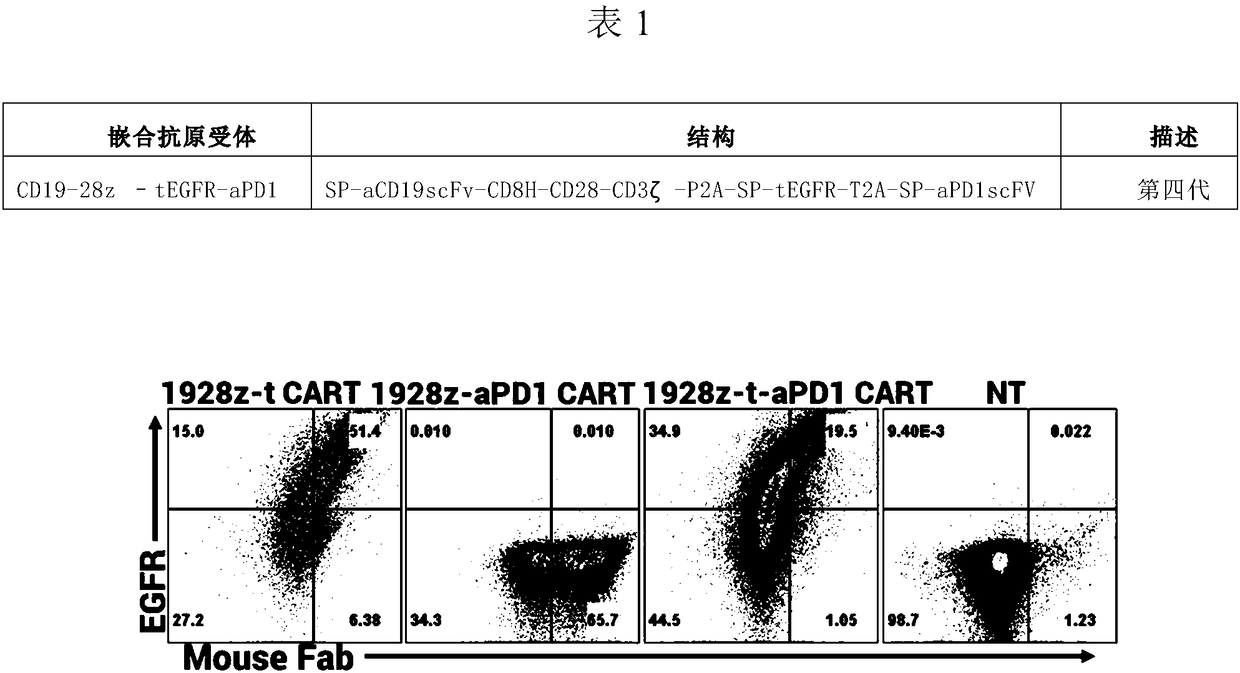
[0081] Example 1: Determination of CD19scFv-CD8α-CD28-CD3ζ-tEGFR-aPD1scFv gene sequence
[0082] 1. From the NCBI website database searched anti-CD19 antibody light chain and heavy chain variable regions, human CD8α hinge region, human CD28 transmembrane region and intracellular region, human CD3ζ intracellular region, anti-PD1 antibody heavy chain and The light chain variable region gene sequence information, these sequences are codon-optimized on the website http: / / sg.idtdna.com / CodonOpt to ensure that the encoded amino acid sequence is more suitable for expression in human cells. The whole gene synthesis of the above sequence was introduced, and different enzyme cutting sites were introduced at the beginning and end to form the complete CD19-28z-tEGFR-aPD1 gene sequence information.
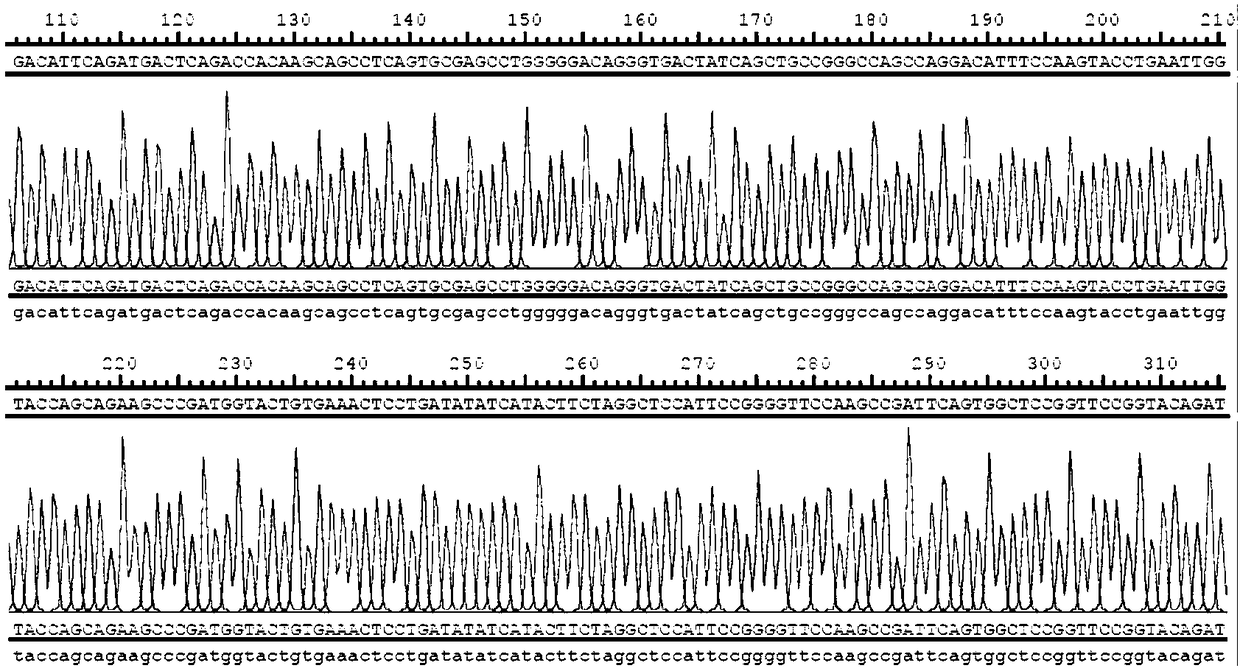
[0083] 2. Recombinant plasmid sequencing
[0084] Send the recombinant plasmid to Shanghai Sangon Biotechnology Co., Ltd. for sequencing, and compare the sequencing results with the simulated...
Embodiment 2
[0088] Example 2: Construction of a viral vector comprising the CD19-28z-tEGFR-aPD1 nucleic acid sequence
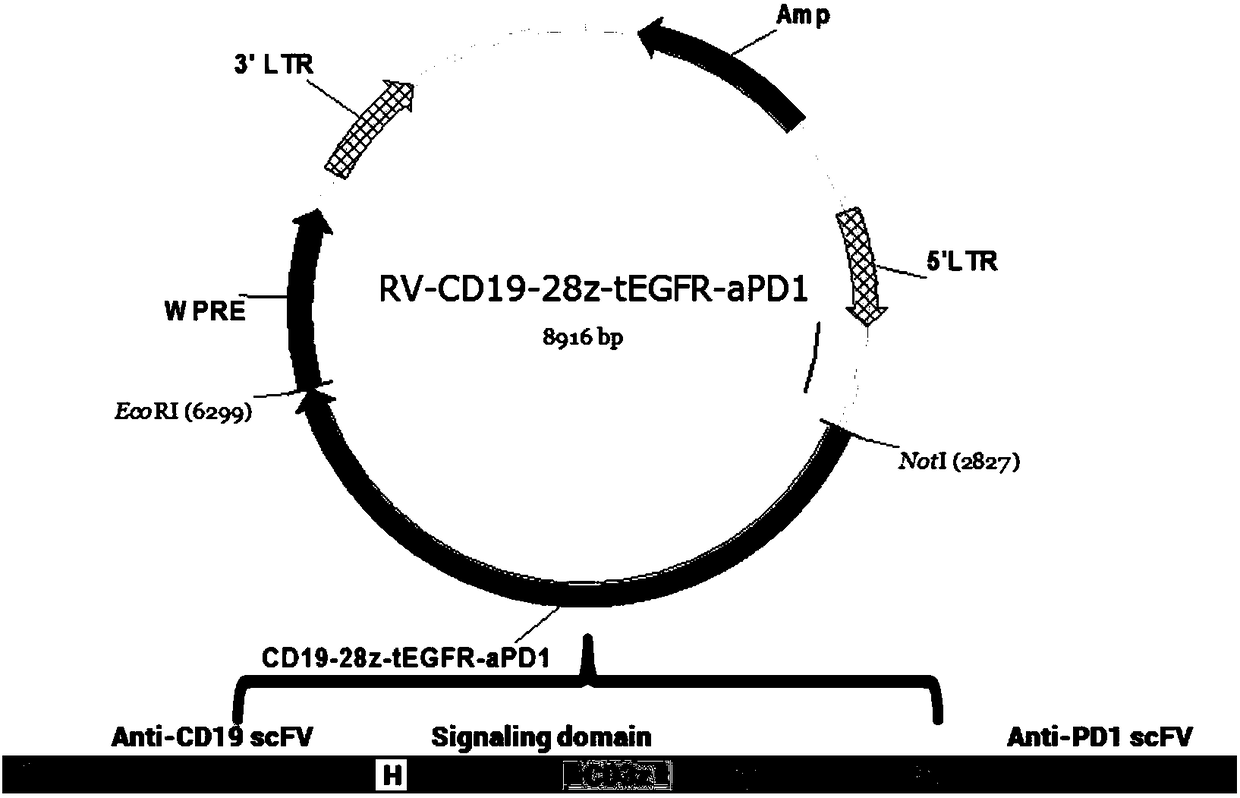
[0089] The CD19-28z-tEGFR-aPD1 nucleotide sequence prepared in Example 1 was double digested with NotI (NEB) and EcoRI (NEB), connected with T4 ligase (NEB) and inserted into the NotI-EcoRI of the retroviral MSCV vector The site was transformed into competent E.coli (DH5α). After the sequencing was correct, the plasmid was extracted and purified using Qiagen’s plasmid purification kit, and the purified plasmid was transfected into 293T cells by the plasmid calcium phosphate method for retrovirus packaging experiments.
[0090] The plasmid map constructed in this example is as follows figure 1 shown. figure 2 The peak diagram of partial sequencing results of the retroviral expression plasmid is shown.
Embodiment 3
[0091] Example 3: Retroviral packaging
[0092] 1. On the first day, the 293T cells should be less than 20 passages and not overgrown. Plate at 0.6*10^6 cells / ml, add 10ml of DMEM medium to a 10cm dish, mix the cells well, and culture overnight at 37 degrees.
[0093] 2. On the second day, the confluence of 293T cells reached about 90% for transfection (usually about 14-18 hours after plating); prepare plasmid complexes, and the amount of various plasmids is RV-CD19-28z-tEGFR-aPD1 (MSCV backbone plasmid ) is 12.5ug, Gag-pol is 10ug, VSVg is 6.25ug, CaCl 2 250ul,H 2 O is 1ml and the total volume is 1.25ml; add HBS equal to the volume of the plasmid complex in another tube, and vortex for 20 seconds while adding the plasmid complex. Gently add the mixture to the 293T dish along the side, incubate at 37°C for 4 hours, remove the medium, wash it with PBS, and add pre-warmed fresh medium again.
[0094] 3. Day 4: 48 hours after transfection, collect the supernatant and filter ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com