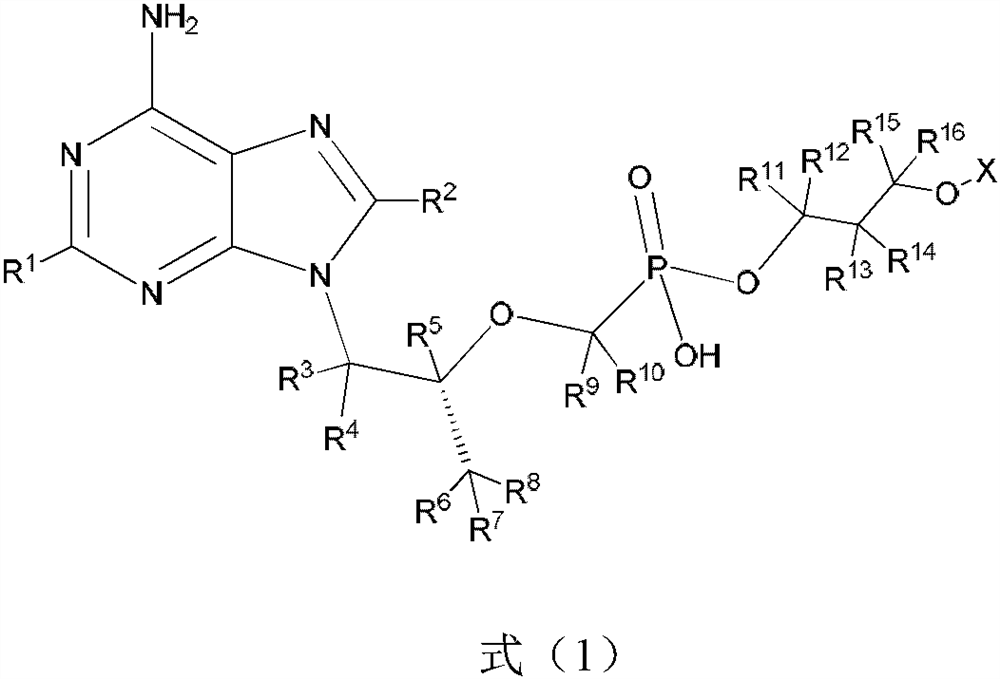
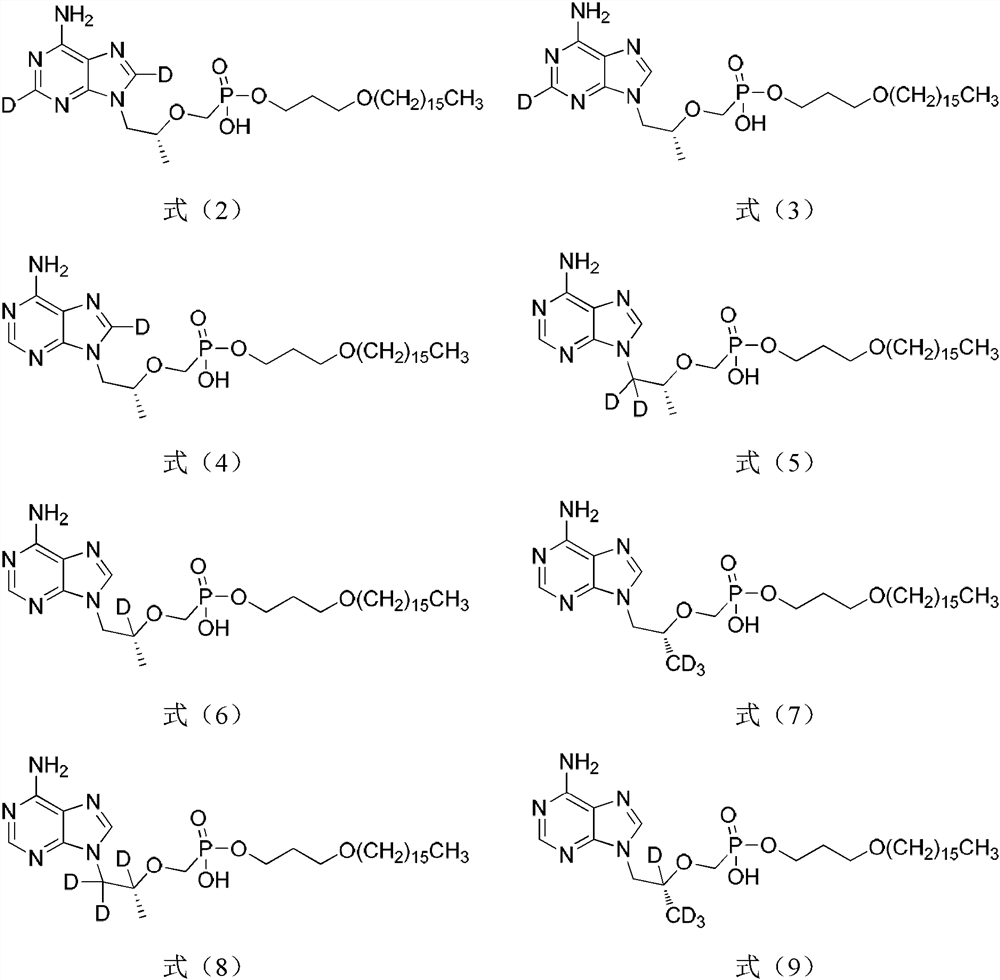
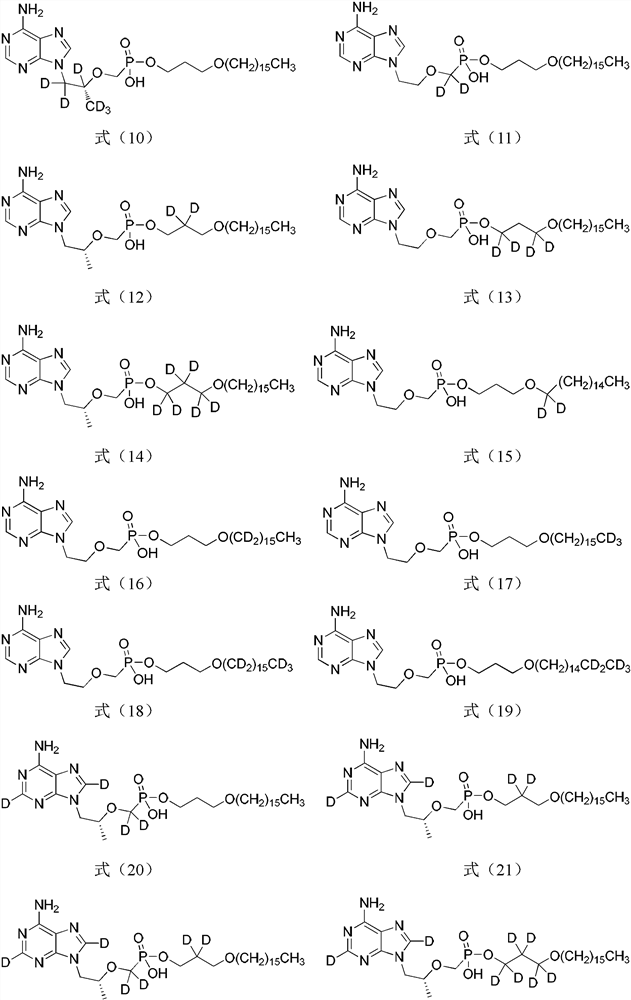
A novel acyclic nucleoside analogue and its pharmaceutical composition
A composition and drug technology, applied in the field of medicine, can solve the problems of nephrotoxicity risk, drug resistance, low absorption efficiency of target cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 Preparation of (R)-9-{2-[(hexadecyloxy-d6-propyl)phosphomethoxy]propyl}adenine, that is, the compound Thing T-1, concrete synthetic steps are as follows:
[0053]
[0054] Step 1 Synthesis of (R)-9-(2-hydroxypropyl)adenine (compound 3).
[0055] Add adenine (4.0g, 29.6mmol) and (R)-propylene carbonate (3.45g, 33.8mmol) into the reaction flask, add 4.5mL DMF to dissolve, heat to 130°C for overnight reaction, TLC detects that the reaction is complete, then cool down To 100°C, add 14mL of toluene and 0.47g of methanesulfonic acid (keep the internal temperature at 100-110°C), then add 11mL of toluene to obtain a homogeneous suspension, gradually cool down to room temperature, then cool down to 0°C for 1 hour, The filtered white solid was vacuum-dried to obtain 5.77 g of product, yield 100%. LC-MS(APCI): m / z=194.3(M+1) + .
[0056] Step 2 Synthesis of diethyl[[(p-toluenesulfonyl)oxy]methyl]phosphate (compound 5).
[0057]Add diethyl hydroxymethyl phosphat...
Embodiment 2
[0066] Example 2 Preparation of (R)-9-{2-[(hexadecyloxy-2-d2-propyl)phosphomethoxy]propyl}adenine, namely Compound T-2, the specific synthesis steps are as follows:
[0067]
[0068] Step 1 Synthesis of diethyl 2-d2-malonate (compound 11).
[0069] Diethyl malonate (4.0g, 25mmol), potassium carbonate (345mg, 2.5mmol) and 15mL of heavy water were added into a microwave reaction vial, sealed and placed in a microwave reactor and heated to 85°C for 45 minutes. After cooling down to room temperature, ethyl acetate was added to extract three times, the organic phases were combined, washed with saturated brine, concentrated and purified by column chromatography to obtain 3.63 g of the target product with a yield of 90.7%. LC-MS(APCI): m / z=163.1(M+1) + .
[0070] Step 2 Synthesis of 2-d2-1,3-propanediol (compound 12).
[0071] Compound 11 (2.26g, 13.94mmol) was added to the reaction flask, dissolved in 50mL of anhydrous tetrahydrofuran, and lithium aluminum hydride (1.06g, ...
Embodiment 3
[0076] Example 3 Preparation of (R)-9-{2-[(hexadecyloxy-1,3-d4-propyl)phosphomethoxy]propyl}adenine, namely Compound T-3, the specific synthesis steps are as follows:
[0077]
[0078] Step 1 Synthesis of 1,3-d4-1,3-propanediol (compound 14).
[0079] Diethyl malonate (1.0g, 6.24mmol) was added to the reaction flask, dissolved in 20mL of anhydrous tetrahydrofuran, under ice bath, deuterated lithium aluminum hydride (0.52g, 12.5mmol) was added in batches, and the addition was completed. The reaction was stirred overnight at room temperature. The reaction was quenched by adding a small amount of decahydrate and sodium sulfate in an ice bath, and the insoluble matter was removed by filtration. The filtrate was concentrated to obtain the crude product of the target product, which was dried in vacuo to obtain 263 mg, with a yield of 52.6%. LC-MS(APCI): m / z=81.1(M+1) + .
[0080] Step 2 Synthesis of 3-hexadecyloxy-1,3-d4-1-propanol (compound 15).
[0081] Add hexadecane b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap